• Easily disseminated or transmitted from person to person
• Result in high death rates and have potential for major public health impact
• Cause public panic and social disruption
• Require special action for public health preparedness
• Anthrax
• Botulism
• Plague
• Smallpox
• Tularemia
• Viral hemorrhagic fevers
• Moderately easy to disseminate
• Result in moderate morbidity rates and low death rates
• Require specific enhancements of CDC’s diagnostic capacity and enhanced disease surveillance
• Brucellosis
• Food/water safety threats
• Glanders
• Melioidosis
• Psittacosis
• Q fever
• Ricin toxin
• Staphylococcal enterotoxin B
• Typhus
• Viral encephalitis
• Emerging infectious diseases that could be engineered for mass dissemination because of their:
• Availability
• Ease of production and dissemination
• Potential for high morbidity and death rates and major health impact
• Nipah virus
• Hantavirus
• Monkeypox
• Severe acute respiratory syndrome (SARS)
• vCJD
• Avian influenza
• Pandemic flu
ISOLATION AND QUARANTINE
Federal law stipulates that isolation and quarantine are mandated for the following diseases caused by biological agents:
• Cholera
• Diphtheria
• Infectious tuberculosis
• Plague
• Smallpox
• Yellow fever
• Viral hemorrhagic fevers (Lassa, Marburg, Ebola, Crimean-Congo, South American, and others)
• Severe acute respiratory syndrome (SARS)
• Novel or re-emergent influenza viruses that may cause a pandemic
TRANSMISSION PRECAUTIONS
In the event of a bioterrorist attack, additional measures may be needed to augment standard nursing precautions (Table 6-2). Depending upon the agent, airborne, droplet, or contact precautions may be required.
| ∗For some VHFs, transmission patterns are not clear and all precautions should be exercised. | ||||
| STANDARD | AIRBORNE | CONTACT | DROPLET | |
|---|---|---|---|---|
| Anthrax, inhalational | X | |||
| Botulism | X | |||
| Brucellosis | X | X | ||
| Cholera | X | |||
| Crimean-Congo | X | X | X | X |
| Ebola | X | X | X | X |
| E. coli | X | X | ||
| Glanders | X | X | X | |
| Hantavirus | X | |||
| Lassa | X | X | X | |
| Marburg | X | X | X | X |
| Melioidosis | X | X | ||
| Plague | X | |||
| Psittacosis | X | X | ||
| Q fever | X | |||
| Ricin toxin | X | |||
| Salmonellosis | X | |||
| Shigellosis | X | X | ||
| Smallpox | X | X | X | |
| Staphylococcal enterotoxin B | X | |||
| Tularemia | X | |||
| Typhus, epidemic | X | X | ||
| Viral encephalitides | X | |||
Airborne Precautions
Airborne transmission involves infected microorganisms or dust particles that remain suspended in the air for long periods of time and can be dispersed widely by air currents. Depending on environmental factors, a susceptible host may inhale particles within the same room or over a longer distance from the source patient. Special air handling and ventilation are required to prevent airborne transmission. In addition to standard precautions, airborne precautions include patient placement in a negative pressure room, use of respiratory protection such as an N95 respirator, and limitation of patient transport.
Droplet Precautions
Droplets can be transmitted from coughing, sneezing, and talking, and during certain procedures such as suctioning and bronchoscopy. Transmission is via the host’s conjunctivae, nasal mucosa, or mouth. Droplets do not remain suspended in the air and as such ventilation is not needed. Do not confuse droplet precautions with airborne transmission. In addition to standard precautions, droplet precautions include placement of the patient in a private room, use of a mask (especially when within 3 feet of the patient), and limited patient transport.
Contact Precautions
Contact is the most frequent mode of transmission of nosocomial infections and is divided into direct- and indirect-contact transmission.
• Direct-contact transmission involves body-to-body contact that results in the physical transfer of microorganisms, such as bathing or turning a patient.
• Indirect-contact transmission involves a susceptible host touching a contaminated object, such as instruments, needles, or dressings or hands that are not washed and gloves that are not changed between patients. Gloves should always be used in contact precautions. Gowns should be worn if there will be body-to-body contact or the patient has diarrhea, an ostomy, or excessive wound drainage. Before leaving the room, remove gloves and gown and thoroughly wash hands; be sure to avoid contact with any contaminated surfaces.
BIOLOGICAL AGENT DESCRIPTIONS
This chapter covers the following biological agents:
• Anthrax (cutaneous, gastrointestinal, and inhalational)
• Botulism
• Brucellosis
• Food safety threats
• Glanders
• Melioidosis
• Plague
• Psittacosis
• Q fever
• Smallpox
• Staphylococcal enterotoxin B
• Tularemia
• Typhus, epidemic
• Viral encephalitides
• Viral hemorrhagic fevers
• Water safety threats
(For information on category C agents, see Chapter 4.)
For each agent, the following information is provided:
• Diagnosis synopsis
• Weaponization
• Transmission/isolation
• Incubation, duration, and mortality
• Patient assessment/recognition
• Clinical diagnostic tests
• Patient management
• Therapy
• Personal safety risk
• Precautions
• Family safety
• Vaccine
• Public health reporting
ANTHRAX

BIOLOGICAL AGENT DESCRIPTION: ANTHRAX
OVERVIEW
Anthrax is a bacterial infection caused by Bacillus anthracis, an encapsulated, gram-positive, spore-forming bacterium. B. anthracis is present in both domestic and wild animals throughout the world and can be transmitted by their meat, wool, or hides. The spores are resistant to heat, UV light, microwave radiation, and many disinfectants. B. anthracis has been classified by the CDC as a category A bioterrorism agent because of its high lethality, hardiness, and ease of weaponization. In 2001 there were 22 cases of anthrax as a result of a bioterrorist attack. In 2002 the U.S. Department of Defense reintroduced the vaccination of military personnel and essential emergency civilians against anthrax.
There are three forms of anthrax:
1. Cutaneous
2. Gastrointestinal
3. Inhalational
Each is discussed separately on the following pages.
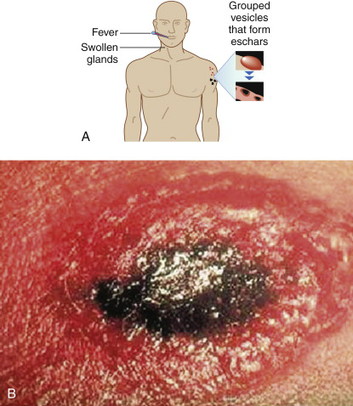
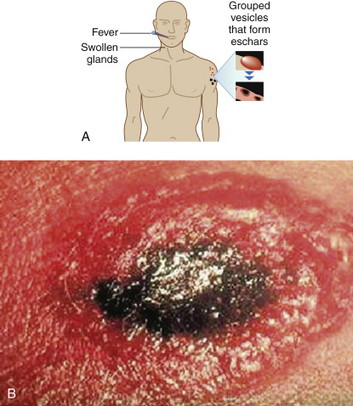
CUTANEOUS ANTHRAX
DIAGNOSIS SYNOPSIS
Although inhalational and gastrointestinal forms of anthrax exist, approximately 95% of all anthrax cases are cutaneous. Before 2001 there had not been a case of cutaneous anthrax reported in the United States since 1992. In the September 11, 2001 attacks, 11 of the 22 cases were cutaneous.
WEAPONIZATION
Cutaneous contact with aerosolized spores.
INCUBATION, ONSET, AND MORTALITY
• Incubation: 1 to 12 days
• Onset: 1 to 2 days
• Mortality: with treatment, <1%; without treatment, 20%
TRANSMISSION/ISOLATION
• Infection through cut or abrasion on the skin or handling infected animal products
• No person-to-person transmission
• Isolation not necessary
PATIENT ASSESSMENT/RECOGNITION
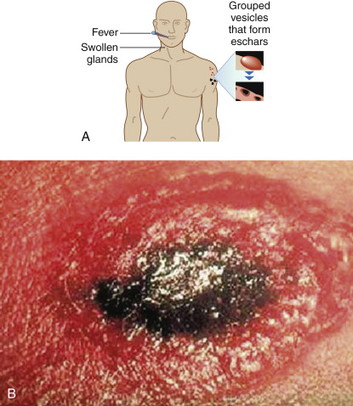
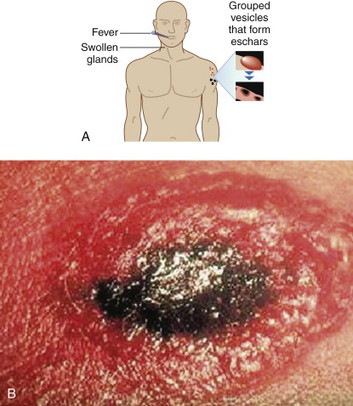
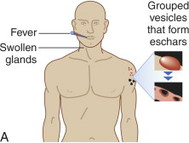 |
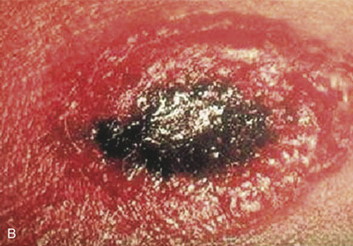 |
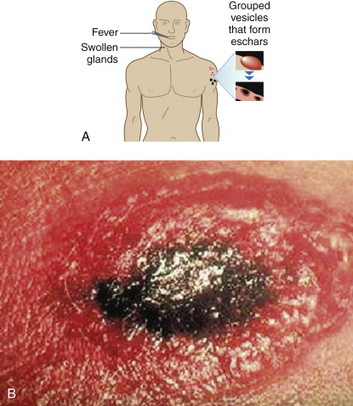
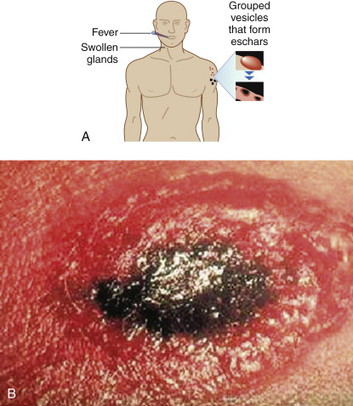
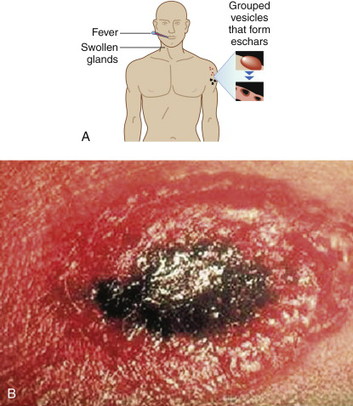
Cutaneous anthrax lesions evolve from pruritic papules to clusters of vesicles to ulcers within 1 to 2 days following exposure of abraded skin or wounds to the spores. The ulcers then develop into depressed black eschar over the next 2 to 5 days. The most common areas affected are the arms, face, and neck. Lymphangitis and painful lymphadenopathy are common.
With antibiotic treatment the death rate for cutaneous anthrax is approximately 1%. However, without treatment it may progress to a systemic form of anthrax with a death rate of approximately 20%. In these cases, the spores introduced into the body are eaten by macrophages and taken to regional lymph nodes, where they germinate into bacteria. Released into the lymph system, they enter the bloodstream, causing septicemia that results in a fatal toxemia.
CLINICAL DIAGNOSTIC TESTS
• Gram stain
• Blood culture
• Polymerase chain reaction (PCR)
PATIENT MANAGEMENT
• Notify your local department of health to inform them of a patient with suspected anthrax and to obtain any additional instructions before performing diagnostic tests.
• Initiate treatment based on a history of exposure or contact, not laboratory test results.
• Obtain specimens for culture; then notify your local laboratory regarding suspected anthrax and inform the laboratory that samples will be sent shortly.
• Do not use extended-spectrum cephalosporins or trimethoprim/sulfamethoxazole because anthrax may be resistant to these drugs.
THERAPY
See Table 6-3 for drug therapy.
| ADULT | CHILDREN | |
|---|---|---|
| Ciprofloxacin | 500-mg oral tablet every 12 hr | 20-30 mg/kg every 12 hr; do not exceed 1 g/day |
| Doxycycline | 100-mg oral tablet every 12 hr | If >45 kg: same as adult If <45 kg: 2.5 mg/kg every 12 hr |
| Amoxicillin | 500-mg oral pill every 8 hr | Depends on body weight |
PERSONAL SAFETY RISK
 Low risk to personal safety.
Low risk to personal safety.PRECAUTIONS
Standard precautions. Avoid direct contact with lesion(s) or lesion drainage.
FAMILY SAFETY/LEAVING WORK
 Low: Cutaneous anthrax is not contagious.
Low: Cutaneous anthrax is not contagious.VACCINE
Vaccine is available.
GASTROINTESTINAL ANTHRAX
DIAGNOSIS SYNOPSIS
Gastrointestinal anthrax is rare; it is caused by consuming contaminated meat products and results in inflammation of the gastrointestinal (GI) tract.
For the purposes of biological warfare, GI anthrax would be placed in the food supply. Some countries have already experimented with this agent; both Japan and the former apartheid government of South Africa created anthrax-laced chocolates. The last reported case of GI anthrax in the United States was in 2000.
WEAPONIZATION
Contamination of food supply.
INCUBATION, ONSET, AND MORTALITY
• Incubation: 1 to 7 days
• Onset: within 7 days
• Mortality: 25% to 60%
TRANSMISSION/ISOLATION
• Consumption of infected/undercooked meat
• No person-to-person transmission
• Isolation not necessary
PATIENT ASSESSMENT/RECOGNITION
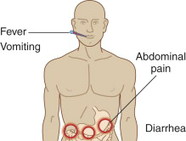 |
Symptoms of gastrointestinal anthrax include fever, anorexia, abdominal pain, ascites, bloody vomit, and diarrhea.
CLINICAL DIAGNOSTIC TESTS
• Gram stain
• Blood culture
PATIENT MANAGEMENT
• Notify your local department of health to inform them of the patient with suspected gastrointestinal anthrax and to obtain any additional instructions before performing diagnostic tests.
• Initiate treatment based on a history of exposure or contact, not laboratory test results.
• Obtain specimens for culture; then notify your local laboratory regarding suspected gastrointestinal anthrax and inform the laboratory that samples will be sent shortly.
• Antimicrobial therapy is critical during the early stages.
• Do not use extended-spectrum cephalosporins or trimethoprim/sulfamethoxazole because anthrax may be resistant to these drugs.
THERAPY
See Table 6-4 for drug therapy.
| ADULT | CHILDREN | |
|---|---|---|
| Ciprofloxacin | 500-mg oral tablet every 12 hr | 20-30 mg/kg every 12 hr; do not exceed 1 g/day |
| Doxycycline | 100-mg oral tablet every 12 hr | If >45 kg: same as adult If <45 kg: 2.5 mg/kg every 12 hr |
| Amoxicillin | 500-mg oral pill every 8 hr | Depends on body weight |
PERSONAL SAFETY RISK
 Low risk to personal safety.
Low risk to personal safety.PRECAUTIONS
Standard precautions.
FAMILY SAFETY/LEAVING WORK
 Low: Gastrointestinal anthrax is not contagious.
Low: Gastrointestinal anthrax is not contagious.VACCINE
Vaccine is available.
INHALATIONAL ANTHRAX
DIAGNOSIS SYNOPSIS
If anthrax were to be weaponized, the most likely method of dispersal would be by aerosol release, and although gastrointestinal and cutaneous forms of anthrax exist, inhalational anthrax would be the most likely to occur. In the 2001 terrorist attacks, 11 of the 22 cases were inhalational, resulting in 5 deaths. Before these attacks, the last reported case of inhalational anthrax was in 1976. A single case of inhalational anthrax may represent a bioterrorist threat and a public health emergency.
Extremely rare in normal circumstances, inhalational anthrax reaches a death rate of nearly 40% if treatment is not started in the prodromal phase.
WEAPONIZATION
Aerosol inhalation.
INCUBATION, ONSET, AND MORTALITY
• Incubation: 1 to 13 days
• Onset: 7 days
• Mortality: approximately 40%
TRANSMISSION/ISOLATION
• No person-to-person transmission
• Isolation not necessary
PATIENT ASSESSMENT/RECOGNITION
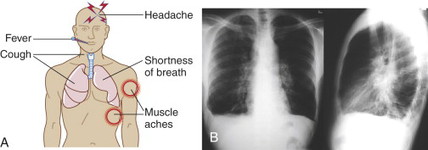 |
Look for nonspecific, flulike symptoms (e.g., low-grade fever, sweats, dry cough, headache, malaise, fatigue, dyspnea, and myalgias) in the first 24 to 48 hours with early chest radiography or computer tomography (CT) demonstrating a widened mediastinum and (often) pleural effusions. Infiltrates may also be present.
Initial symptoms are followed by a sudden onset of high fever and severe respiratory distress along with cyanosis, hypotension, shock, respiratory failure, and sudden death.
CLINICAL DIAGNOSTIC TESTS
• Chest x-ray (CXR)
• Gram stain
• Blood culture
• PCR
PATIENT MANAGEMENT
• Notify your local department of health to inform them of the patient with suspected inhalational anthrax and to obtain any additional instructions before doing diagnostic tests.
• Initiate treatment based on a history of exposure or contact, not laboratory test results.
• Obtain specimens for culture and notify your local laboratory regarding suspected anthrax and inform them that samples will be sent shortly.
• Early antibiotic therapy and cardio-respiratory support is critical; supportive therapy includes pleural effusions.
• Do not use extended-spectrum cephalosporins or trimethoprim/sulfamethoxazole because anthrax may be resistant to these drugs.
THERAPY
See Table 6-5 for drug therapy.
| ADULT | CHILDREN | |
|---|---|---|
| Ciprofloxacin | 500-mg oral tablet every 12 hr | 20-30 mg/kg every 12 hr; do not exceed 1 g/day |
| Doxycycline | 100-mg oral tablet every 12 hr | If >45 kg: same as adult If <45 kg: 2.5 mg/kg every 12 hr |
| Amoxicillin | 500-mg oral pill every 8 hr | Depends on body weight |
PERSONAL SAFETY RISK
 Low risk to personal safety.
Low risk to personal safety.PRECAUTIONS
Standard precautions.
FAMILY SAFETY/LEAVING WORK
 Low: Inhalational anthrax is not contagious.
Low: Inhalational anthrax is not contagious.VACCINE
Vaccine is available.
BOTULISM

BIOLOGICAL AGENT DESCRIPTION: BOTULISM
DIAGNOSIS SYNOPSIS
Botulism is caused by a group of paralytic neurotoxins produced by the spore-forming, anaerobic, gram-positive bacillus Clostridium botulinum. Intoxication can occur naturally from contaminated foods or rarely as a result of wound or intestinal colonization in humans. There are seven types of botulinum toxins, A through G, although only A, B, E, and F have been implicated in the poisoning of humans. When used as a biological weapon, botulinum toxin is most likely to be dispersed as an aerosol for inhalation, although it could be added to the food or water supply. Botulinum toxins are, by weight, the most toxic agents known, with an LD50∗ of only 0.001 mcg/kg. Botulinum toxin irreversibly binds to the presynaptic cholinergic neuromuscular junction, blocking acetylcholine release and manifesting as a symmetrical, progressive, descending flaccid paralysis after an 18- to 36-hour incubation period (occasionally up to several days). Multiple cranial nerve palsies are evident early in the course of the disease. Botulinum toxins are odorless and colorless in solution. Five minutes of heating the toxin above 85° C will inactivate the toxin. All materials suspected of containing toxin must be handled with caution. Before the shipment of any botulism-associated specimen, the designated laboratory must be notified and approved by the state health department.
∗The Lethal dose that results in the death of 50% of the subjects who are exposed to it.
WEAPONIZATION
• Aerosol inhalation
• Contamination of food and water supply
INCUBATION, ONSET, AND MORTALITY
• Incubation: 18 to 36 hours
• Onset: 6 hours to 2 weeks
• Mortality: overall, 5% to 10%; age older than 60 years, 30%
TRANSMISSION/ISOLATION
• No person-to-person transmission
• Isolation not necessary
PATIENT ASSESSMENT/RECOGNITION
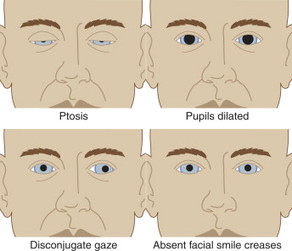 |
The hallmark symptoms of botulism are difficulty speaking, seeing, and/or swallowing in combination with ptosis on exam. Also look for dilated (enlarged) and/or fixed pupils, diminished gag reflex, tongue weakness, and arm/leg weakness. The mouth may appear dry (xerostomia). The patient is afebrile.
CLINICAL DIAGNOSTIC TESTS
Sensitivity testing: brain scan, spinal fluid examination, nerve conduction test, and Tensilon test for myasthenia gravis (rules out similar diseases, such as Guillain-Barré syndrome).
PATIENT MANAGEMENT
• Diagnosis primarily based on medical history and physical examination
• Supportive care and passive immunization with equine botulinum antitoxin (available from the CDC)
THERAPY
• Equine botulinum antitoxin
• Dose: dilute 10-ml vial, 1:10 in 0.9% saline solution; deliver IV slowly
PERSONAL SAFETY
 Low risk to personal safety.
Low risk to personal safety.PRECAUTIONS
Standard precautions.
FAMILY SAFETY/LEAVING WORK
 Low: Botulism is not contagious.
Low: Botulism is not contagious.PROPHYLAXIS/VACCINE
Equine botulinum antitoxin.
BRUCELLOSIS

BIOLOGICAL AGENT DESCRIPTION: BRUCELLOSIS
DIAGNOSIS SYNOPSIS
Brucellosis is a systemic infection characterized by an undulant (intermittent) fever pattern. Typically a zoonotic infection of farm animals, the disease is produced in humans by infection with the gram-negative coccobacilli of the genus Brucella. Only four species of Brucella cause infection in humans: B. melitensis (goats and sheep), B. ovis (sheep and goats), B. suis (pigs), B. abortus (cattle), and rarely B. canis (dogs). B. suis and B. melitensis are the most common cause of brucellosis in humans. Weaponization of brucella is likely because of its ease of transmission by aerosol, and it is known to have been weaponized by several countries. During World War II the United States developed brucellosis as a biological weapon and dispersed it via bombs. Aerosolized brucella is highly infectious. In naturally occurring cases, human infection usually is caused by ingestion of contaminated dairy foods or infected animals (e.g., sheep, cattle, goats, or hogs) or through skin wounds.
WEAPONIZATION
Aerosol inhalation.
INCUBATION, ONSET, AND MORTALITY
• Incubation: 5 days to 6 months
• Onset: within 8 weeks
• Mortality: less than 2%
TRANSMISSION/ISOLATION
• Person-to-person transmission is rare.
• Isolation is not necessary.
CLINICAL DIAGNOSTIC TESTS
• PCR
• Antibody-based antigen detection systems
• Enzyme-linked immunosorbent assay (ELISA)
• Blood or bone marrow cultures
PATIENT ASSESSMENT/RECOGNITION
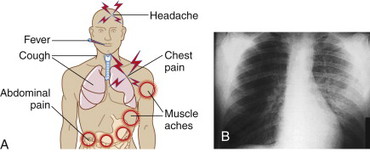 |
Brucellosis produces both an acute and a debilitating chronic illness. Typical acute systemic symptoms are nonspecific and flulike, and include undulant (intermittent) fevers, headache, chills, profuse sweating, malaise, myalgias, arthralgias, back pain, fatigue, anorexia, and irritability. GI symptoms are common and include nausea, vomiting, diarrhea, ileitis, colitis, and hepatitis. Other findings include bone pain caused by focal infection, pleuritic chest pain, and cough. Adenopathy, pharyngitis, and rash occur more commonly in children. Infection may progress to include the central nervous system (CNS) (meningitis) or the heart (endocarditis). Osteoarticular infections of the spine with paravertebral abscesses are common.
Medium and large joints are also commonly infected. Genitourinary involvement can lead to pyelonephritis, cystitis, and epididymo-orchitis. Infection with Brucella can lead to chronic painful symptoms lasting months. Death rates are less than 2%, and are often associated with endocarditis.
PATIENT MANAGEMENT
• Initiate therapy immediately.
• Administer doxycycline and rifampin in combination for 6 weeks to prevent reoccurring infection.
• Early treatment is critical; severity and duration of illness are dependent on timing of treatment.
THERAPY
See Table 6-6. Continue therapy for 6 weeks.
| ADULTS | CHILDREN | |
|---|---|---|
| Doxycycline | 100 mg twice per day, oral | 100 mg twice per day, oral |
| Rifampin | 600 mg/day | 15-20 mg/kg/day in one to two doses; maximum 600-900 mg/day |
PERSONAL SAFETY RISK
 Low risk to personal safety.
Low risk to personal safety.PRECAUTIONS
Standard precautions.
FAMILY SAFETY/LEAVING WORK
 Low: Brucellosis is not contagious.
Low: Brucellosis is not contagious.VACCINE
None available.
FOOD SAFETY THREATS

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


