- Urine production – the concept of glomerular filtration rate (GFR)
- Metabolic production of urea and creatinine
- Urea and creatinine as markers of renal disease
- Non-renal causes of increased plasma urea
- Estimating GFR from plasma creatinine concentration
- Use of eGFR in identifying and staging chronic kidney disease
Measurement of the serum or plasma concentration of urea and creatinine are included in the most commonly requested profile of blood chemistry, ‘urea and electrolytes’ (U&E). They are both tests of kidney function, though plasma creatinine is the more specific in this regard. Estimated glomerular filtration rate (e-GFR) is a calculated parameter based on plasma creatinine concentration that is now recommended for diagnosis and monitoring of chronic kidney disease (CKD), a common slowly progressive condition that currently affects close to 10% of the adult UK population1. Diabetes, hypertension, obesity and advancing age are the major risk factors that predispose individuals to CKD. With an ever ageing population, and increasing prevalence of both diabetes and obesity, CKD is a major and growing health problem in the UK and other developed countries around the world. eGFR allows early identification, long before symptoms develop; medical intervention at this stage can prevent or slow progression of CKD as well as reduce the risk of the cardiovascular disease (hypertension, heart attacks, strokes) that CKD can be associated with.
Normal physiology
Formation of urine by the kidneys – what is GFR?
As the site of production of the hormones renin, erythropoietin and calcitriol – that regulate respectively blood pressure, red blood cell production and absorption of dietary calcium from the gut, – the kidneys have significant and disparate endocrine function. But the principle function of the kidneys is formation of urine from filtered blood.
Urine is the vehicle for excretion of many unwanted or toxic products of metabolism (including urea and creatinine) as well as substances (e.g. sodium, potassium, hydrogen ions) that are essential to life but present in quantity surplus to the body’s immediate needs. By their ability to vary over wide limits the amount, chemical composition and pH of urine, the kidneys serve a major role in maintaining constant volume, chemical composition and pH of both blood and the fluid within all tissue cells. This constant internal environment is essential for normal cell function; life depends upon it. Thus urine is not just a vehicle for excretion; its formation has life-preserving homeostatic significance.
The two kidneys are located one on either side of the vertebral column at the level of the two lowest ribs, partially projecting below the rib cage. They are deep within the abdominal cavity in the small of the back. Adult kidneys are around 12 cm long and each is supplied with blood via its own single renal artery that branches directly from the main vessel (the aorta) descending from the heart. Blood leaves each kidney via a single renal vein that drains directly to the inferior vena cava, one of two major vessels that convey blood back to the heart.
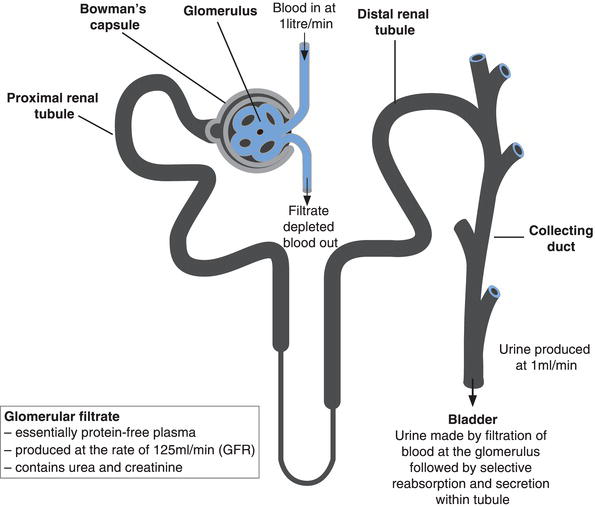
The functional unit of the kidney – where urine is formed – is the nephron (Figure 5.1) a tiny tubular structure whose length is measured in millimetres. There are around 1 million nephrons in each kidney. The nephron comprises two distinct elements: the glomerulus and the renal tubule. The glomerulus is a knot of blood capillaries conveying arterial blood that has flowed from the renal artery via multiple sub-dividing smaller bore vessels to the smallest (arterioles), and finally into the glomerulus. This knot of tiny blood vessels (capillaries), the glomerulus, is sited in a round, ‘globular’ structure called Bowman’s capsule, which forms the first part of the renal tubule, called the proximal renal tubule.
Formation of urine begins at the glomerulus. Net filtration pressure within the capillary bed (due principally to arterial blood pressure) forces water and all other blood borne substances of medium and low molecular weight (including urea and creatinine) to pass from blood flowing through the glomerulus into the Bowman’s capsule. The process is called glomerular filtration and the fluid formed, which is the precursor of urine, is called the glomerular filtrate. The glomerulus is not sufficiently permeable to allow the passage of blood cells or large molecular weight proteins into Bowman’s capsule so that the glomerular filtrate is essentially protein-depleted blood plasma.
The rate at which this filtrate is formed by the totality of nephrons is the glomerular filtration rate (GFR). In health the GFR is around 125 ml/minute or 180 L per day. If there were no way of reabsorbing the product of glomerular filtration, the whole of the blood volume (around 5–6 L) would be lost within a few hours! In fact the composition and volume of the glomerular filtrate is greatly modified as it passes from the Bowman’s capsule through the length of each renal tubule. Around 80% of the filtered water and essential constituents of blood, for example, electrolytes, amino acids, glucose etc., are reabsorbed back into the blood during passage through the proximal renal tubule. There is further capacity for some substances to be reabsorbed to blood and secreted from blood into the tubule during final regulation of urine composition in the distal renal tubule. The exact amount of water, electrolytes and nutrients reabsorbed depends upon the body’s requirement at the time, but urine, the final product of first filtration, then tubular reabsorption and secretion, is produced at the rate of around 1 ml/minute, that is 1.5 L/day. The urine produced by each nephron flows into a system of collecting ducts which coalesce, thereby connecting all nephrons to the ureter, the muscular tube that propels urine from the kidney to the bladder.
Clinical significance of GFR
The glomerular filtration rate (GFR) is a parameter of prime clinical importance because it defines kidney function. All those with loss of kidney function, whatever its cause, have reduced GFR. There is good correlation between GFR and severity of kidney disease, and GFR begins to fall very early in development of CKD, long before symptoms are evident. The rapidity at which GFR decreases distinguishes acute kidney disease/injury (AKI), formerly called acute renal failure, from CKD, formerly called chronic renal failure. AKI progresses very rapidly over a matter of hours or days, but is potentially reversible; CKD progresses much more slowly over months, years or even decades, but is irreversible. Although by no means inevitable, both AKI and CKD may progress to effective renal failure (sometimes called end-stage renal disease), at which point survival depends on renal replacement therapies. These include short-term continuous dialysis in the case of AKI, and either life-long intermittent dialysis or kidney transplantation, in the case of CKD.
What are urea and creatinine?
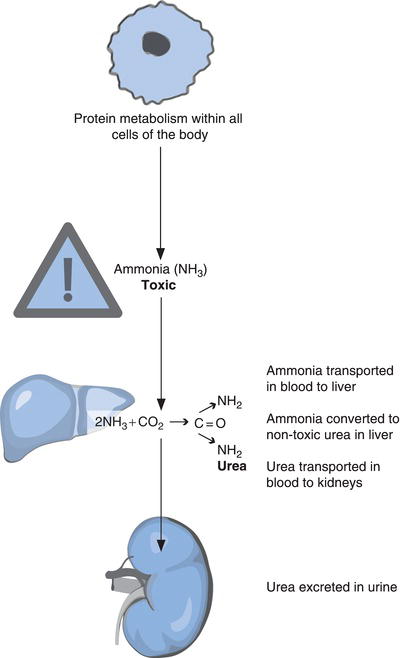
Normal cellular metabolism of proteins and amino acids results in production of ammonia (NH3). This toxic by-product of metabolism is transported in the blood to the liver where it is converted to non-toxic urea by a series of enzyme mediated reactions known as the urea cycle (Figure 5.2).
Urea itself has no metabolic function; as a waste product of normal metabolism it must be eliminated from the body. Once synthesised in the liver it is transported via the blood to the kidney where it is excreted in urine.
Creatinine has a similar fate. Like urea it is a waste product of metabolism, more precisely muscle metabolism; it is formed from a substance called creatine that is involved in producing the energy required for muscle cell contraction. Creatinine is released to blood from muscle cells and transported to the kidneys where it is excreted in urine along with urea. If the ability of the kidneys to excrete urea and creatinine is compromised, they accumulate in blood; the plasma concentration of both rises. Of the two tests, plasma creatinine concentration is the most reliable marker of kidney function in part because, as will become clear, increase in plasma urea concentration is not confined to those individuals suffering kidney disease; it is a less specific marker of renal disease.
Renal handling of urea and creatinine
Both urea and creatinine are filtered from blood at the glomerulus. Since both are waste products of metabolism there is no reason for either to be reabsorbed. However a very small proportion of filtered urea is reabsorbed to blood and this tendency to reabsorption is greater if the urea concentration of the filtrate is particularly high. No creatinine is reabsorbed, but a small amount of that appearing in urine (5–10%) is secreted from blood into the tubule. Notwithstanding these two minimal effects, the amount of urea and creatinine excreted in urine and therefore the amount remaining in blood is dependent on the glomerular filtration rate; as GFR falls, so does urea and creatinine excretion. As excretion falls, blood levels rise.
Laboratory measurement of plasma/serum urea and creatinine
Patient preparation
Ideally, patients should be on a meat free diet during the 24 hours prior to creatinine measurement, because cooked meat is a potential source of exogenous creatinine. It is important for most accurate assessment of kidney function that only the creatinine produced within the body is measured.
Timing of sample
Blood may be sampled at any time of the day.
Sample requirements
These two tests are usually performed as part of the U&E screen. Around 5 ml of venous blood is required for U&E. Measurement may be made on either the serum or plasma recovered from a blood sample. If local policy is to use serum then blood must be collected into a collection tube without anticoagulant. Plasma estimation requires collection into a collection tube containing the anticoagulant lithium heparin.
Effects of storage
Unlike the other parameters measured in a U&E profile, urea and creatinine concentration remains stable when blood is stored for up to 24 hours at room temperature.
Interpretation of results
Approximate reference ranges
Plasma/serum urea concentration | 2.5–7.8 mmol/L |
Plasma/serum creatinine concentration | 55–105 µmol/L |
[Note: There are gender and age differences for plasma/serum creatinine because concentration depends on total muscle mass. In general plasma concentration among females and the elderly, who have relatively low muscle mass, tends to be at the lower end of the reference range whereas that for non-elderly adult males (i.e. those with greatest muscle mass) tends to be at the high end of the reference range.]
Old age is associated with physiological deterioration in renal function, so that urea increases very gradually with increasing age. The tendency for creatinine concentration to increase due to reduced renal excretion in old age is offset by decreased production due to age related reduction in muscle mass.
Critical values
Plasma/serum urea | > 28.0 mmol/L |
Plasma/serum creatinine | > 450 µmol/L |
Causes of reduced plasma/serum urea concentration
- Pregnancy is normally associated with an increased GFR and therefore increased rate of urea excretion; pregnant women typically have lower plasma/serum urea concentration than non-pregnant women.
- Low protein diet. Urea synthesis is a function of amino acid and protein metabolism, which in turn is affected by dietary intake of proteins. Those on a low protein diet synthesise less urea than those on a normal diet.
- Liver disease. Urea synthesis occurs in the liver. Although this function is not usually affected in mild to moderate liver disease, liver failure is associated with decreased urea synthesis and as a consequence accumulation in blood of oxic ammonia.
Causes of a reduced plasma/serum creatinine
- Pregnancy is associated with increased GFR and therefore increased excretion of creatinine.
- Creatinine is produced by muscle cells. Any disease associated with significant decrease in muscle mass (e.g. muscular dystrophy, severe malnutrition) may result in abnormally low plasma creatinine concentration.
Causes of an increased plasma/serum urea and creatinine concentration
Renal causes
Both plasma urea and plasma creatinine concentration are raised if glomerular filtration rate (GFR), that is renal function, is significantly reduced. The glomerulus is analogous to any other filtration system where the rate of filtration depends on three factors:
- The rate at which the liquid (blood in this case) to be filtered is presented to the filter.
- The patency of the filter (a ‘blocked’ filter will result in a slower filtration rate).
- Any opposing pressure on the other side of the filter, reducing filtration rate.
Extending this analogy to the many causes of renal disease allows a simplified classification of renal disease to pre-renal (reduced blood flow to kidneys), renal (damage to the filter itself) and post-renal (obstruction to urine flow) renal disease. Table 5.1 describes such an approach emphasising that a low GFR and therefore raised concentration of urea and creatinine is a feature of all causes of renal dysfunction.
These tests provide no information about the cause of renal dysfunction. However they are good markers of renal disease progression, because as renal function (GFR) falls, both urea and creatinine concentration rise.
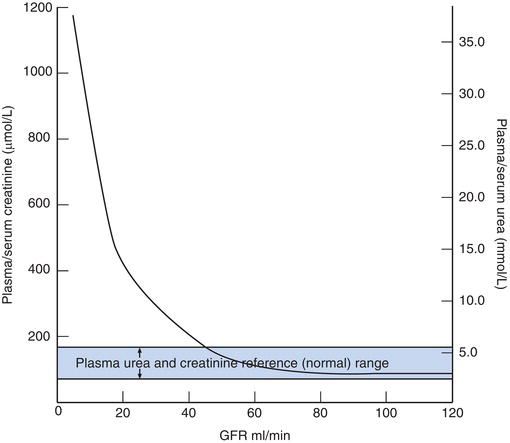
It is important to emphasise that a normal urea and creatinine concentration does not exclude early chronic kidney disease, because concentration of both urea and creatinine only begin to rise reliably above the reference range after considerable loss of renal function. Figure 5.3 illustrates this point; plasma urea and creatinine concentrations remain within their respective reference range until the GFR has fallen to around 40 ml/minute, less than 50% of its normal value.
Table 5.1 A classification of some common causes of renal disease – irrespective of the cause GFR is reduced.
| Pre-renal renal disease | Renal disease | Post-renal renal disease |
| Reduced GFR due to reduction in blood volume being presented for filtration. Kidneys structurally normal (at least initially) but functionally compromised. Causes: Any condition that results in marked reduction in blood volume or blood pressure:
| Reduced GFR due to disease related damage to ‘filter’ (glomerulus). In simplistic terms ‘blocked filter’. Kidney structurally damaged and therefore functionally compromised. Causes:
| Reduced GFR due to blockage on the distal side of the nephron opposing filtration pressure. Kidneys structurally normal but functionally compromised. Causes: Any condition that obstructs urine flow:
|
Figure 5.3 Relationship between glomerular filtration rate (GFR) and plasma concentration of urea and creatinine. Note plasma concentration of urea and creatinine remains normal until GFR is reduced by more than 50%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree