Chapter 5 Anaemias and the haemoglobinopathies
RELEVANT ANATOMY AND PHYSIOLOGY
The blood
An average man has, circulating in his body, approximately 5.6 L of blood, 1 L in lungs, 3 L in systemic venous circulation and 1 L in heart and arteriolar circulation. A woman has approximately 4.5 L of blood and a newborn baby, conversely, has only 250–300 mL. Blood volume can be calculated for either a man or woman by calculating 7% of body weight in kilograms (Martini 2005).
Plasma
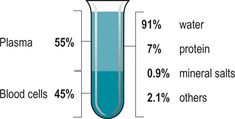
Plasma (Fig. 5.1) is the straw coloured fluid which remains after the blood cells have been removed. It is composed of 90–95% water and plasma proteins, which are formed in the liver and have many uses: nutrients, electrolytes, hormones, enzymes and waste products.
Plasma and interstitial fluid encompass most of the volume of extracellular fluid in the body. As water, ions and small solutes are continually exchanged between interstitial fluid, capillary walls and plasma their composition is very similar (Martini 2005). The characteristic straw colour of plasma is formed from bilirubin – the main bile pigment derived from haemoglobin breakdown (Stables & Rankin 2005). Plasma contains dissolved proteins, which because of their large size and shape, cannot cross capillary walls. The main plasma proteins are albumin, globulins and fibrinogen.
Fibrinogen accounts for approximately 4% of plasma proteins and is essential in the clotting of blood and can be converted into insoluble fibrin. The remaining 1% is composed of specialized plasma proteins whose roles vary widely (Martini 2005).
Erythrocytes
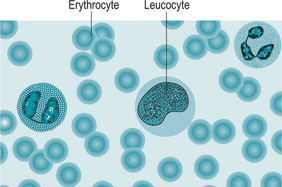
Erythrocytes (Fig. 5.2) or red blood cells are non-nucleated bi-concave discs. This unusual shape has an important effect on the function of erythrocytes. Large surface area to volume allows rapid exchange of oxygen from the cell to tissues. When passing through narrow blood vessels erythrocytes can form stacks one behind each other to smooth the flow without restriction. Additionally they are able to bend and flex when entering and squeezing through small arterioles and capillaries.
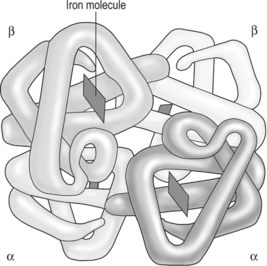
Oxygen is carried in erythrocytes bound to the protein haemoglobin (Fig. 5.3). A haemoglobin molecule consists of four polypeptide chains, with a ‘haem’ portion at the centre of each chain. Each ‘haem’ portion is a deep red pigment that contains one iron atom bound to one oxygen molecule. Haemoglobin molecules can bind up to four oxygen molecules (Box 5.1).
If the body detects low levels of oxygen, for example at high altitude, or through anaemia,the release of erythropoietin is increased; this in turn stimulates the bone marrow to produce more erythroblasts. If the demand is excessive, immature cells such as reticulates may be released prematurely into the circulation (Hand 2001).
Leucocytes
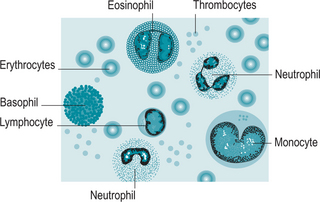
Leucocytes (Fig. 5.4) (white blood cells) are nucleated cells, are much larger than red blood cells and are involved in protecting the body against disease. Leucocytes comprise 1% of the circulating blood volume. They have the ability of leaving the blood vessels to invade diseased tissues. Leucocytes are classified according to whether they are granular or agranular.
Agranular leucocytes, monocytes and lymphocytes, have round or kidney-shaped nuclei and cytoplasm that lacks any granules. Monocytes become tissue macrophages. There are three different types of lymphocytes. T cells are involved in cell-mediated immunity whereas B cells are primarily responsible for humoral immunity (relating to antibodies). Some T lymphocytes act as natural killer cells and are responsible for the detection and destruction of abnormal tissue cells, for example viruses and cancers.
Thrombocytes
Thrombocytes (Fig. 5.5) (platelets) are small, sticky, granular blood cell fragments that play an essential role in the arrest of bleeding or haemostasis. They are the first line of defence against damage to blood vessels; they adhere to any defects and assist in formation of a platelet plug and a fibrin clot during the clotting process. Platelets live for 9–12 days before being destroyed by phagocytes, mainly in the spleen. There are on average 350 000 platelets/μL of circulating blood.
The blood thus contains a number of cells which give it a vital role in the normal functioning of the body (Table 5.1).
From Martini 2005.
Classification of blood
ABO blood groups
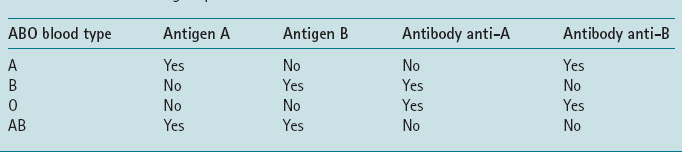
There are four main blood types A, B, AB, O (Table 5.2).
When red blood cells carrying one or both antigens are exposed to the corresponding antibodies, they agglutinate; that is, clump together. People usually have antibodies against those red cell antigens that they lack. If a person is transfused with blood of a different group than their own, it is the reaction between the donor’s red cell and the recipient’s serum that causes the potentially fatal side-effects.
Rhesus factor
Individuals either have, or do not have, the Rhesus factor (or RhD antigen) on the surface of their red blood cells. A person is either Rhesus positive (does have the Rhesus antigen) or Rhesus negative (does not have the antigen). The antibody anti-D does not occur normally in the serum of Rhesus negative blood. If a person receives a Rhesus positive blood transfusion or if during childbirth a woman who is Rhesus negative receives red blood cells from a Rhesus positive baby, antibodies are produced, and if in a subsequent pregnancy the fetus is Rhesus positive, maternal antibodies will cause haemolysis of fetal red blood cells, which causes anaemia and jaundice or if severe, may even cause death (Box 5.2).
PHYSIOLOGICAL CHANGES IN THE CARDIOVASCULAR SYSTEM AND BLOOD IN PREGNANCY
Heart size increases to cope with increased cardiac output. Upward displacement of the diaphragm by the enlarging uterus causes the heart to be displaced to the left and rotated anteriorly.
ANAEMIA
Iron requirements during pregnancy
Extra iron is required by the body during pregnancy; the total iron demands range between 580 and 1340 mg, and of that, up to 1050 mg will be lost at birth (Hillman 1996). In early pregnancy, the requirement is approximately 2.5 mg/day and this increases to around 6.6 mg/day in the third trimester. A normal diet contains 15–20 mg of iron/day in developed countries, and 3–10% is absorbed mainly from the duodenum. In a healthy woman, the loss of iron daily is 1–2 mg (Jordan & McOwat 2002).
Anaemia
Anaemia in pregnancy
Iron deficiency anaemia
Iron deficiency anaemia occurs in 23% of pregnant women in developed countries and in 52% of women in developing countries (WHO et al 2001).
Predisposing factors
Poor nutrition or absorption of nutrients
The Western diet with refined foods can be deficient in B vitamins, especially folic acid. A vegetarian diet may need to be supplemented with iron, vitamins B and D. Conversely, some micronutrients are hazardous if overused in pregnancy, for example, vitamin A, found in liver, which is associated with birth defects, such as cleft palate and heart defects (IVAC 2006).
Maternal nutrition even in developed countries can be less than optimum, particularly where women with low income, as a result of poor education, minimum wage employment or unemployment, are unable to provide themselves with an adequate diet. It is also well known that there are many misconceptions of what constitutes a healthy diet and health education advice may be misunderstood (Blincoe 2006).
The National Institute for Clinical Excellence (NICE) published, through their Screening committee, a policy position stating that all pregnant women should be offered a test for anaemia. This should be considered a fundamental part of antenatal care. NICE plans to publish a routine antenatal care guideline in late 2007 (National Screening Policy Position 2006).
Women on low incomes may not take in enough calories to meet the energy demands of pregnancy, and consequently, the intake of micronutrients may also be insufficient (Jordan & McOwat 2002). Poor nutrition can lead to the woman being less able to cope with the rigours of labour. This in turn may lead to low birthweight infants with a poor ability to cope with illness or disease.
Treatment of the underlying disease can prevent or reverse the anaemia. Chronic diseases such as Crohn’s disease are difficult to treat and patients may exhibit intermittent anaemia that varies with their condition.
Excessive or prolonged blood loss
Chronic infections such as pyelonephritis, malaria, or intestinal parasites may also lead to anaemia (Box 5.3 and Fig. 5.6). Malaria is not endemic in the UK, but approximately 2000 cases occur every year in travellers returning from malaria-endemic countries. Pregnant women should be advised against travel into such areas (Health Protection Agency 2007).
Box 5.3 Hookworm
Infestation with hookworm can provoke iron deficiency and iron deficiency anaemia (Fig. 5.6). The hookworm is a parasitic worm. It thrives in warm climates, including African and American countries. The hookworm enters the body through the skin, through the soles of bare feet, where it then migrates to the small intestines and attaches itself to the villi. The hookworm damages the villi, resulting in blood loss, and they produce anticoagulants that promote continued bleeding. Each worm can provoke the loss of up to 0.25 mL of blood/day. Because of international travel and the migrant population, hookworm is increasingly likelyto be seen in the UK.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree