Antimicrobial Agents
Objectives
2 Describe the signs and symptoms of the common adverse effects seen with antimicrobial therapy.
6 Explain the major actions and effects of drugs used to treat infectious diseases.
Key Terms
pathogenic (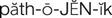 ) (p. 727)
) (p. 727)
antibiotics (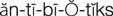 ) (p. 727)
) (p. 727)
prophylactic antibiotics (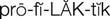 ) (p. 728)
) (p. 728)
nephrotoxicity (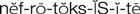 ) (p. 729)
) (p. 729)
ototoxicity (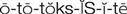 ) (p. 729)
) (p. 729)
gram-positive microorganisms (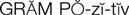
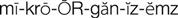 ) (p. 735)
) (p. 735)
gram-negative microorganisms (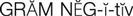 ) (p. 736)
) (p. 736)
hypoprothrombinemia (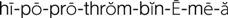 ) (p. 736)
) (p. 736)
thrombophlebitis (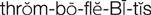 ) (p. 736)
) (p. 736)
penicillinase-resistant penicillins (
 ) (p. 743)
) (p. 743)
Antimicrobial Agents
![]() http://evolve.elsevier.com/Clayton
http://evolve.elsevier.com/Clayton
Antimicrobial agents are chemicals that eliminate living microorganisms that are pathogenic to the patient. Antimicrobial agents may be of chemical origin, such as the sulfonamides, or they may be derived from other living organisms. Those derived from other living microorganisms are called antibiotics; for example, penicillin was first derived from the mold Penicillium notatum. Most antibiotics used today are harvested from large colonies of microorganisms, which are then purified and chemically modified into semisynthetic antimicrobial agents. The chemical modification makes the antibiotic more effective against specific pathogenic organisms. Antimicrobial agents are often first classified according to the type of pathogen to be destroyed, such as bacteria (antibacterial agents), fungus (antifungal agents), or virus (antiviral agents). The antimicrobial agents are then subdivided by chemical families into drug classes such as the penicillins, tetracyclines, and aminoglycosides.
The selection of the antimicrobial agent must be based on the sensitivity of the pathogen and the possible toxicity to the patient. If at all possible, infecting organisms should first be isolated and identified. Culture and sensitivity tests should be completed to identify the infective organism and determine the antibiotic to which the infecting organism is most sensitive. However, if clinically indicated, a patient may be started on a broad-spectrum antibiotic regimen until the infection organism is identified and its drug sensitivity is determined. The antimicrobial therapy is then started based on the sensitivity results and the clinical judgment of the health care provider. In the inpatient setting, however, it is routine to obtain specimens of the infecting organism from infected sites (e.g., blood, urine, wound) and then start therapy immediately with one or more antimicrobial agents that are most likely to stop the infection. This is known as empirical treatment. When the cultures identify the pathogen and the sensitivity tests indicate which antimicrobial agent will be most effective, the most effective antimicrobial agent is continued (or started, if not empirically prescribed) and all other antimicrobial agents are discontinued. Discontinuation of therapy of unneeded antimicrobial agents helps prevent the development of resistant organisms and reduce health care costs.
The use of prophylactic antibiotics is recommended for patients at risk for developing infective endocarditis before dental, gastric, and genitourinary surgery, and other invasive procedures. The American Heart Association (Wilson et al, 2007) guidelines recommend prophylactic antibiotic treatment for patients with cardiac conditions that put them at highest risk for infection, such as prosthetic cardiac valve; previous infective endocarditis; congenital heart disease (CHD); unrepaired, cyanotic CHD, including palliative shunts and conduits; congenital heart defects completely repaired with prosthetic material or device—whether placed by surgery or by catheter intervention—during the first 6 months after the procedure; repaired congenital heart defects with residual defects at the site or adjacent to the site of prosthetic patch or prosthetic device; and cardiac transplantation.
 Nursing Implications for Antimicrobial Therapy
Nursing Implications for Antimicrobial Therapy
Nurses must consider the entire patient when administering and monitoring antimicrobial therapy. It is essential that the nurse be knowledgeable about the drugs, including physiologic parameters for monitoring expected therapeutic activity and adverse effects. It is important to teach the individual with an infection the basic principles of self-care, which will enhance the recovery process, and measures to prevent the spread of infection. With communicable diseases, exposed individuals must be contacted for follow-up testing and appropriate treatment.
Assessment
History of Current Infection.
What symptoms are described by the patient? Which of the symptoms described potentially relate to an infectious process? Extend questioning to help the patient focus—for example, when did the symptoms begin? Have they worsened? Is there fever, night sweats, malaise, chronic fatigue, weight loss, arthralgia, cough (type of secretions), diarrhea, painful urination, nausea, vomiting, lesions or skin rash, discharge, or drainage? Is there any swelling, pain, or heat in a particular area or discharge from a site? Has the patient been treated previously for a similar infection? Ask questions specific to the body systems affected by the infection (e.g., pain and burning with urination for a urinary tract infection [UTI]). Determine patient’s current living environment and review the institution policy. Many patients residing in long-term care facilities are screened for methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE).
When treating patients with sexually transmitted infections (STIs), ask about the number of sexual partners, sexual orientation, and use of precautions during intercourse. (See Chapter 41 for further details.)
Past History.
Ask the patient about previously treated conditions. Which treatments were used, and what was the response to therapy? Focus on areas that may impinge on antimicrobial therapy, such as reduced renal or hepatic function, immunocompromised conditions, blood dyscrasias, partial deafness, and gastrointestinal (GI) complaints.
Allergies.
Is the patient allergic to certain medicines, dust, weeds, foods, or other environmental substances?
Medication History
Physical Examination
Psychosocial.
For an individual with a serious communicable disease, assess the response and adaptive processes used to cope with the disease and its treatments.
Laboratory and Diagnostic Studies
Assessments During Antimicrobial Therapy.
Read each drug monograph for specific common and serious adverse effects, and individualize the assessments for the drugs prescribed. Nausea, vomiting, diarrhea, allergies, anaphylaxis, nephrotoxicity, hepatotoxicity, ototoxicity, hematologic dyscrasias, secondary infection, and photosensitivity are found with recurring frequency in the antimicrobial drug monographs.
• Nausea, vomiting, and diarrhea: These conditions are the “big three” adverse effects associated with antimicrobial drug therapy. When they occur, gather data such as the following:
1 Did the patient have a history of nausea, vomiting, or diarrhea before starting the drug therapy?
2 How soon after starting the medication did the symptoms start?
3 Since starting the medication, has the diet or water source changed in any way?
• Nephrotoxicity: Assess nephrotoxicity through an increasing BUN and creatinine, decreasing urine output, decreasing urine specific gravity, casts or protein in the urine, frank blood or smoky-colored urine, or red blood cells (RBCs) in excess of 0 to 3 RBCs/high-power field (HPF; see Table 42-1) on the urinalysis report.
1 Ask specifically about any history of blood disorders diagnosed and treatments prescribed.
4 Has the patient received blood cell stimulator drugs such as Epogen or Neupogen?
5 Does the patient have any bleeding disorders, such as hemophilia or thrombocytopenia?
Implementation
• Always monitor for phlebitis when antimicrobials are administered intravenously.
• Administer antimicrobials as prescribed on the time schedule established.
Nausea, Vomiting, and Diarrhea.
When drug therapy causes nausea and vomiting, the health care provider may elect to give the antibiotic with food to decrease irritation, even though absorption may be slightly decreased, or may choose to switch to a parenteral dosage form. When reporting any incidence of nausea and vomiting, all significant data should be collected and reported. Administer prescribed antiemetics or antidiarrheal agents (see also Chapters 34 and 35).
Secondary Infection.
Secondary infection may occur in patients receiving broad-spectrum antibiotic therapy, particularly those who are immunosuppressed.
Monitor for the development of symptoms of secondary infection, and notify the health care provider if this occurs. Initiate prescribed treatment consistent with the cause of the symptoms. Obtain cultures as ordered, and administer additional antimicrobials effective against the new organism. Instruct the patient to minimize exposure to people known to have an infection and to practice good personal hygiene measures.
Allergies and Anaphylaxis.
Closely monitor all patients—particularly those with histories of allergies, asthma, or rhinitis and those who are taking multiple drug preparations—for an allergic response during antimicrobial therapy. All patients should be watched carefully for possible allergic reactions for at least 20 to 30 minutes after administration of a medication. However, some drug reactions may not occur for several days.
Hold the prescribed antimicrobial medication if the person reports a possible allergy; share all information obtained with the health care provider, who will decide whether to administer the drug. Older adults, because of the physiologic changes of aging, require close observation for therapeutic response and for drug toxicity. Learn the location of the hospital emergency cart and the procedure for summoning it. In the event of suspected anaphylaxis, summon the health care provider and the emergency cart immediately.
Although a serious reaction may occur with the first administration of a drug, repeated exposures to a previously sensitized substance can be fatal. Respond immediately to any signs of reaction, including swelling, redness, or pain at the site of injection, hives, nasal congestion and discharge, wheezing progressing to dyspnea, pulmonary edema, stridor, and sternal retractions.
When symptoms of an allergic response occur, follow hospital protocol, which will usually include the following steps:
Nephrotoxicity.
Report abnormal laboratory results relating to kidney function (BUN, serum creatinine levels). Maintain an accurate intake and output record; report declining output, or output below 30 mL/hr in the adult patient.
Many antimicrobial agents are potentially nephrotoxic (e.g., aminoglycosides, tetracyclines, vancomycin). Concomitant therapy with diuretics enhances the likelihood of toxicity, particularly in the older or debilitated patient. When renal function is impaired, most drug dosages must be decreased or alternate drug therapy used.
Hepatotoxicity.
Several drugs to be studied in this chapter are potentially hepatotoxic (e.g., isoniazid, sulfonamides). The liver is active in the metabolism of many drugs, and drug-induced hepatitis may occur. The actual liver damage may occur shortly after exposure to the pharmacologic agent or may not appear for several weeks after initial exposure. The symptoms of hepatotoxicity are anorexia, nausea, vomiting, jaundice, hepatomegaly, splenomegaly, and abnormal liver function test results (e.g., elevated bilirubin, AST, ALT, GGT, alkaline phosphatase levels, increased PT). Patients with preexisting hepatic disease such as cirrhosis or hepatitis will require lower dosages of drugs metabolized by the liver.
Ototoxicity.
Report preexisting hearing impairment or symptoms of developing hearing deficits to the health care provider and initiate orders prescribed. Provide for patient safety if tinnitus or dizziness accompanies the symptoms of hearing impairment.
Blood Dyscrasias.
Individualize care to the type of blood dyscrasia present. When hypoprothrombinemia is present, the usual treatment is administration of vitamin K. Serious and possibly fatal bone marrow suppression may occur after therapy is initiated with some antibiotics (e.g., chloramphenicol). Monitor for signs and symptoms such as sore throat, fatigue, elevated temperature, small petechial hemorrhages, and bruises on the skin, and if present, report these symptoms immediately.
Photosensitivity.
This adverse effect is seldom evident during hospitalization. It is more commonly seen in ambulatory practice.
When the drug monograph mentions this as a potential serious adverse effect, the nurse should provide health teaching to prevent its occurrence. Instruct the patient to avoid exposure to sunlight and ultraviolet light (e.g., sun lamps, suntanning beds); wear long-sleeved clothing, a hat, and sunglasses; and apply sunscreen to exposed skin when going out into the sunlight.
Medication History
 Patient Education and Health Promotion
Patient Education and Health Promotion
The following basic principles of patient care should not be overlooked when treating a patient with infections:
Medications
Fostering Health Maintenance
Written Record.
Enlist the patient’s aid in developing and maintaining a written record of monitoring parameters (e.g., list presenting symptoms: cough with a large amount of phlegm, wound drainage, temperature, exercise tolerance) (see Patient Self-Assessment Form for Antibiotics on the Evolve![]() Web site at http://evolve.elsevier.com/Clayton). Complete the Premedication Data column for use as a baseline to track response to drug therapy. Ensure that the patient understands how to use the form, and instruct the patient to take the completed form to follow-up visits. During follow-up visits, focus on issues that will foster adherence to the therapeutic interventions prescribed.
Web site at http://evolve.elsevier.com/Clayton). Complete the Premedication Data column for use as a baseline to track response to drug therapy. Ensure that the patient understands how to use the form, and instruct the patient to take the completed form to follow-up visits. During follow-up visits, focus on issues that will foster adherence to the therapeutic interventions prescribed.
Drug Therapy for Infectious Disease
Drug Class: Aminoglycosides
Actions
Aminoglycoside antibiotics kill bacteria primarily by inhibiting protein synthesis. Other mechanisms of action have not yet been fully defined.
Uses
The aminoglycosides are used primarily against gram-negative microorganisms that cause UTIs, meningitis, wound infections, and life-threatening septicemias. They are the mainstays in the treatment of nosocomial gram-negative infections (e.g., Acinetobacter spp., Citrobacter spp., Enterobacter spp., Escherichia coli, Klebsiella spp., Providencia spp., Pseudomonas spp., Salmonella spp., and Shigella spp.). Kanamycin and neomycin may also be used before surgery to reduce the normal flora content of the intestinal tract.
Therapeutic Outcome
The primary therapeutic outcome expected from aminoglycoside therapy is elimination of bacterial infection.
 Nursing Implications for Aminoglycosides
Nursing Implications for Aminoglycosides
Premedication Assessment
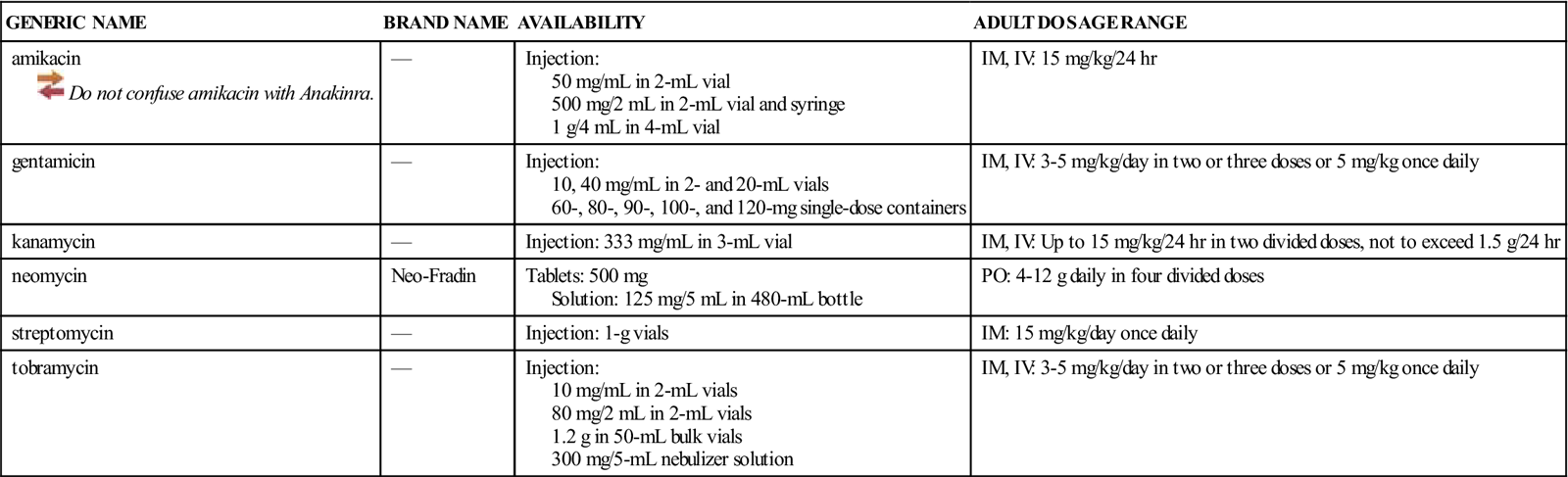
Availability, Dosage, and Administration
See Table 46-1.
![]() Table 46-1
Table 46-1
| GENERIC NAME | BRAND NAME | AVAILABILITY | ADULT DOSAGE RANGE |
| amikacin | — | Injection: 50 mg/mL in 2-mL vial 500 mg/2 mL in 2-mL vial and syringe 1 g/4 mL in 4-mL vial | IM, IV: 15 mg/kg/24 hr |
| gentamicin | — | Injection: 10, 40 mg/mL in 2- and 20-mL vials 60-, 80-, 90-, 100-, and 120-mg single-dose containers | IM, IV: 3-5 mg/kg/day in two or three doses or 5 mg/kg once daily |
| kanamycin | — | Injection: 333 mg/mL in 3-mL vial | IM, IV: Up to 15 mg/kg/24 hr in two divided doses, not to exceed 1.5 g/24 hr |
| neomycin | Neo-Fradin | Tablets: 500 mg Solution: 125 mg/5 mL in 480-mL bottle | PO: 4-12 g daily in four divided doses |
| streptomycin | — | Injection: 1-g vials | IM: 15 mg/kg/day once daily |
| tobramycin | — | Injection: 10 mg/mL in 2-mL vials 80 mg/2 mL in 2-mL vials 1.2 g in 50-mL bulk vials 300 mg/5-mL nebulizer solution | IM, IV: 3-5 mg/kg/day in two or three doses or 5 mg/kg once daily |
Compatibilities.
DO NOT mix other drugs in the same syringe or infuse together with other drugs. See Drug Interactions on p. 733 for incompatibilities.
Rate of Infusion.
Check with the hospital laboratory regarding timing of aminoglycoside blood level tests. The rate of infusion is quite important for aminoglycoside antibiotics, especially if pre- and post-infusion serum levels are being measured. Consult with a pharmacist or see the individual package literature for proper infusion rate. After levels have been determined, assess whether results are normal or toxic.
Monitoring
Serious Adverse Effects
Sensory
Ototoxicity.
Damage to the eighth cranial nerve can occur as a result of aminoglycoside therapy. This may initially be manifested by dizziness, tinnitus, and progressive hearing loss. Continue to observe patients for ototoxicity after therapy has been discontinued. These adverse effects may appear several days later. Assess each patient for difficulty in walking unaided and assess the level of hearing daily. Intentionally speak softly. Note if the patient is aware that anything is being said. Take particular notice of the patient who repeatedly asks, “What did you say?” or who starts talking more loudly or progressively increases the volume on the television or radio.
Genitourinary
Nephrotoxicity.
Monitor urinalysis and kidney function tests for abnormal results. Report increasing BUN and creatinine levels, decreasing urine output or decreasing specific gravity (despite amount of fluid intake), casts or protein in the urine, frank blood or smoky-colored urine, or RBCs in excess of 0 to 3 RBCs/HPF (see Table 42-1) on the urinalysis report.
Drug Interactions
Nephrotoxic Potential.
Cephalosporins, enflurane, vancomycin, and diuretics, when combined with aminoglycosides, may increase the nephrotoxic potential. Monitor the urinalysis and kidney function test findings for abnormal results.
Ototoxic Potential.
Aminoglycosides, when combined with ethacrynic acid, torsemide, bumetanide, and furosemide, may increase ototoxicity. Therefore, nursing assessments for tinnitus, dizziness, and decreased hearing should be done regularly every shift.
Neuromuscular Blockade.
Aminoglycoside antibiotics in combination with skeletal muscle relaxants may produce respiratory depression.
Check the anesthesia records of postoperative patients to determine if skeletal muscle relaxants such as succinylcholine or pancuronium bromide were administered during surgery.
The nurse should monitor and assess the respiratory rate, depth of respirations, and chest movement and report apnea immediately. Because these effects may be seen for up to 48 hours after administration of skeletal muscle relaxants, continue monitoring respirations, pulse, and blood pressure beyond the usual postsurgical vital signs routine.
Heparin.
The aminoglycoside gentamicin and heparin are physically incompatible. DO NOT mix together before infusion.
Beta-Lactam–Type Antibiotics (Penicillins, Cephalosporins).
These drugs rapidly inactivate aminoglycoside antibiotics. DO NOT mix together or administer together at the same IV site.
Drug Class: Carbapenems
Actions
The carbapenems are extremely potent broad-spectrum antibiotics resistant to beta-lactamase enzymes secreted by bacteria. They act by inhibiting bacterial cell wall synthesis.
Uses
Imipenem-cilastatin (Primaxin) is a combination product containing a carbapenem antibiotic called imipenem and cilastatin, an inhibitor of the renal dipeptidase enzyme dehydropeptidase I. Cilastatin has no antimicrobial activity; it prevents the inactivation of imipenem by the renal enzyme. It is used for the treatment of lower respiratory tract and intra-abdominal infections, infections of the urinary tract, bones, joints, and skin, gynecologic infections, endocarditis, and bacterial septicemia caused by gram-negative or gram-positive organisms. A primary therapeutic role of imipenem-cilastatin is in the treatment of severe infections caused by multiresistant organisms and in mixed anaerobic-aerobic infections, primarily those involving intra-abdominal and pelvic sepsis in which Bacteroides fragilis is a common pathogen. It should be used in combination with antipseudomonal agents because of resistance of Pseudomonas cepacia and P. aeruginosa to imipenem.
Meropenem is a carbapenem antibiotic that has a chemical structure that protects it against dehydropeptidase I, so that cilastatin is not necessary. It has a broad spectrum of activity similar to that of imipenem, but it is more active against Enterobacteriaceae and less active against gram-positive bacteria. It is used alone by the IV route for the treatment of intra-abdominal infections caused by Escherichia coli, Klebsiella pneumoniae, P. aeruginosa, B. fragilis, and Peptostreptococcus spp. It is also used alone intravenously to treat bacterial meningitis caused by Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis.
Ertapenem is a carbapenem antibiotic that has a chemical structure that protects it against dehydropeptidase I, so that cilastatin is not necessary. It has a broad spectrum of activity and is approved to treat infections caused by aerobic and anaerobic gram-positive and gram-negative bacteria causing complicated intra-abdominal infections, skin and skin structure infections, community-acquired pneumonia, UTIs (including pyelonephritis), and acute pelvic infections. It is effective against susceptible strains of Staphylococcus aureus, S. agalactiae, S. pneumoniae, Streptococcus pyogenes, E. coli, K. pneumoniae, Moraxella catarrhalis, H. influenzae, Bacteroides spp., Clostridium spp., and Peptostreptococcus spp.
Doripenem is a single agent that has a gram-negative and gram-positive spectrum similar to imipenem and meropenem combined. It is used to treat complicated intra-abdominal infections caused by E. coli, K. pneumoniae, P. aeruginosa, Bacteroides caccae, B. fragilis, B. thetaiotaomicron, B. uniformis, B. vulgatus, Streptococcus intermedius, S. constellatus, and Peptostreptococcus micros. It is also approved for use in complicated UTIs caused by E. coli, including cases with concurrent bacteremia, K. pneumoniae, Proteus mirabilis, P. aeruginosa, and Acinetobacter baumannii.
Therapeutic Outcome
The primary therapeutic outcome expected from carbapenem therapy is elimination of bacterial infection.
 Nursing Implications for Carbapenems
Nursing Implications for Carbapenems
Premedication Assessment
1 Obtain baseline assessments of presenting symptoms.
2 Record temperature, pulse, respirations, blood pressure, and hydration status.
3 Assess for and record any gastric symptoms before initiating therapy.
4 Assess for any allergies. Ask specifically about penicillin and cephalosporin allergies.
7 Ask whether there is a history of seizure activity before initiating therapy.
Availability, Dosage, and Administration
See Table 46-2.
![]() Table 46-2
Table 46-2
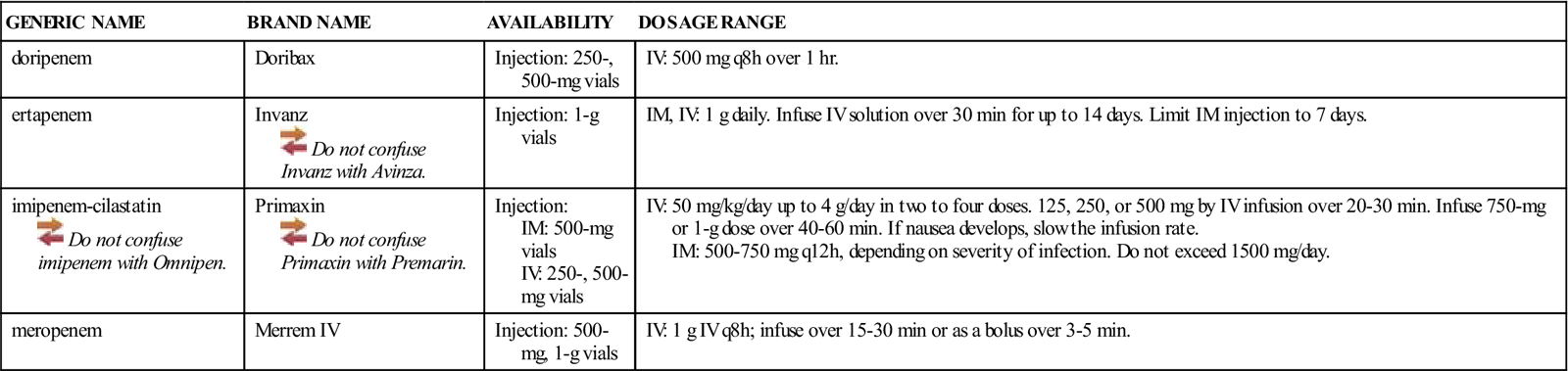
| GENERIC NAME | BRAND NAME | AVAILABILITY | DOSAGE RANGE |
| doripenem | Doribax | Injection: 250-, 500-mg vials | IV: 500 mg q8h over 1 hr. |
| ertapenem | Invanz | Injection: 1-g vials | IM, IV: 1 g daily. Infuse IV solution over 30 min for up to 14 days. Limit IM injection to 7 days. |
| imipenem-cilastatin | Primaxin | Injection: IM: 500-mg vials IV: 250-, 500-mg vials | IV: 50 mg/kg/day up to 4 g/day in two to four doses. 125, 250, or 500 mg by IV infusion over 20-30 min. Infuse 750-mg or 1-g dose over 40-60 min. If nausea develops, slow the infusion rate. IM: 500-750 mg q12h, depending on severity of infection. Do not exceed 1500 mg/day. |
| meropenem | Merrem IV | Injection: 500-mg, 1-g vials | IV: 1 g IV q8h; infuse over 15-30 min or as a bolus over 3-5 min. |
Hypersensitivity.
Although these antibiotics are carbapenems rather than penicillins or cephalosporins, they also contain a beta-lactam nucleus. Cross-hypersensitivity may develop between these classes. Complete a history of hypersensitivity before starting therapy. If an allergic reaction to a carbapenem occurs, discontinue the infusion. Serious reactions may require epinephrine and other emergency measures.
Monitoring
Common Adverse Effects
Gastrointestinal
Severe Diarrhea.
Severe diarrhea may develop from using carbapenems. Blood and mucus in the stool may also be present. This may be an indication of drug-induced pseudomembranous colitis and should be reported immediately. Withhold the next dose of antibiotic until the health care provider gives approval for administration.
Neurologic
Dizziness.
Provide for patient safety during episodes of dizziness; report for further evaluation.
Confusion, Seizures.
Seizure activity—including myoclonic activity, focal tremors, confusional states, and other seizures—has been reported with the carbapenems. These episodes occur most commonly in patients with histories of previous seizure activity and renal impairment.
Perform a baseline assessment of the patient’s degree of alertness and orientation to name, place, and time before initiating therapy. Make regularly scheduled subsequent mental status evaluations, and compare findings. Report alterations in consciousness.
Implement seizure precautions. Make sure that the patient continues with anticonvulsant therapy. If seizures develop, provide for patient safety and then record the exact time of seizure onset and duration of each phase, a description of the specific body parts involved, and any progression in the affected parts. Describe automatic responses during the clonic phase: altered, jerky respirations or frothy salivation, dilated pupils and any eye movements, cyanosis, diaphoresis, or incontinence.
Vascular
Phlebitis.
Carefully assess patients for thrombophlebitis. Inspect the IV area frequently when providing care; inspect visually during dressing changes and whenever the IV is changed to a new site. Report redness, warmth, tenderness to touch, and edema in the affected part; if the lower extremities are affected, dorsiflexion of the foot may cause pain in the calf area (Homans’ sign). Compare the affected limb with the unaffected limb.
Drug Interactions
Probenecid.
Probenecid inhibits the urinary excretion of carbapenems. Do not administer probenecid concurrently.
Valproic Acid.
Carbapenem antibiotics may produce clinically significant reductions in valproic acid levels, which may lead to a loss of seizure control. If any carbapenem is administered with valproic acid, serum valproic acid levels should be monitored frequently.
Ganciclovir.
Concurrent administration of ganciclovir and imipenem-cilastatin has resulted in an increased incidence of seizures. Avoid concurrent use if at all possible.
Admixture Compatibility.
The carbapenems require special care regarding mixing and administration.
Drug Class: Cephalosporins
Actions
The cephalosporins are chemically related to the penicillins and have a similar mechanism of activity. The cephalosporins act by inhibiting cell wall synthesis in bacteria. The cephalosporins may be divided into groups, or “generations,” based primarily on antimicrobial activity. The first-generation cephalosporins have effective activity against gram-positive microorganisms (S. aureus, S. epidermidis, S. pyogenes, S. pneumoniae) and relatively mild activity against gram-negative microorganisms (E. coli, K. pneumoniae, P. mirabilis). Second-generation cephalosporins have somewhat increased activity against gram-negative bacteria but are much less active than the third-generation agents, which are generally less active than first-generation agents against gram-positive cocci, although they are much more active against penicillinase-producing bacteria. Some of the third-generation cephalosporins also are active against P. aeruginosa, a potent gram-negative microorganism. Fourth-generation cephalosporins are broad-spectrum agents, with both gram-negative and gram-positive coverage. Ceftaroline is a new fourth-generation cephalosporin that shows promise in treating methicillin resistant staphylococcus aureus (MRSA) and vancomycin-resistant Staphylococcus aureus (VRSA).
Uses
Cephalosporins may be used with caution as alternatives when patients are allergic to the penicillins, unless they are also allergic to the cephalosporins. The cephalosporins are used for certain urinary and respiratory tract infections, abdominal infections, bacteremia, meningitis, and osteomyelitis.
Therapeutic Outcome
The primary therapeutic outcome expected from cephalosporin therapy is elimination of bacterial infection.
 Nursing Implications for Cephalosporins
Nursing Implications for Cephalosporins
Premedication Assessment
Availability, Dosage, and Administration
See Table 46-3.
![]() Table 46-3
Table 46-3
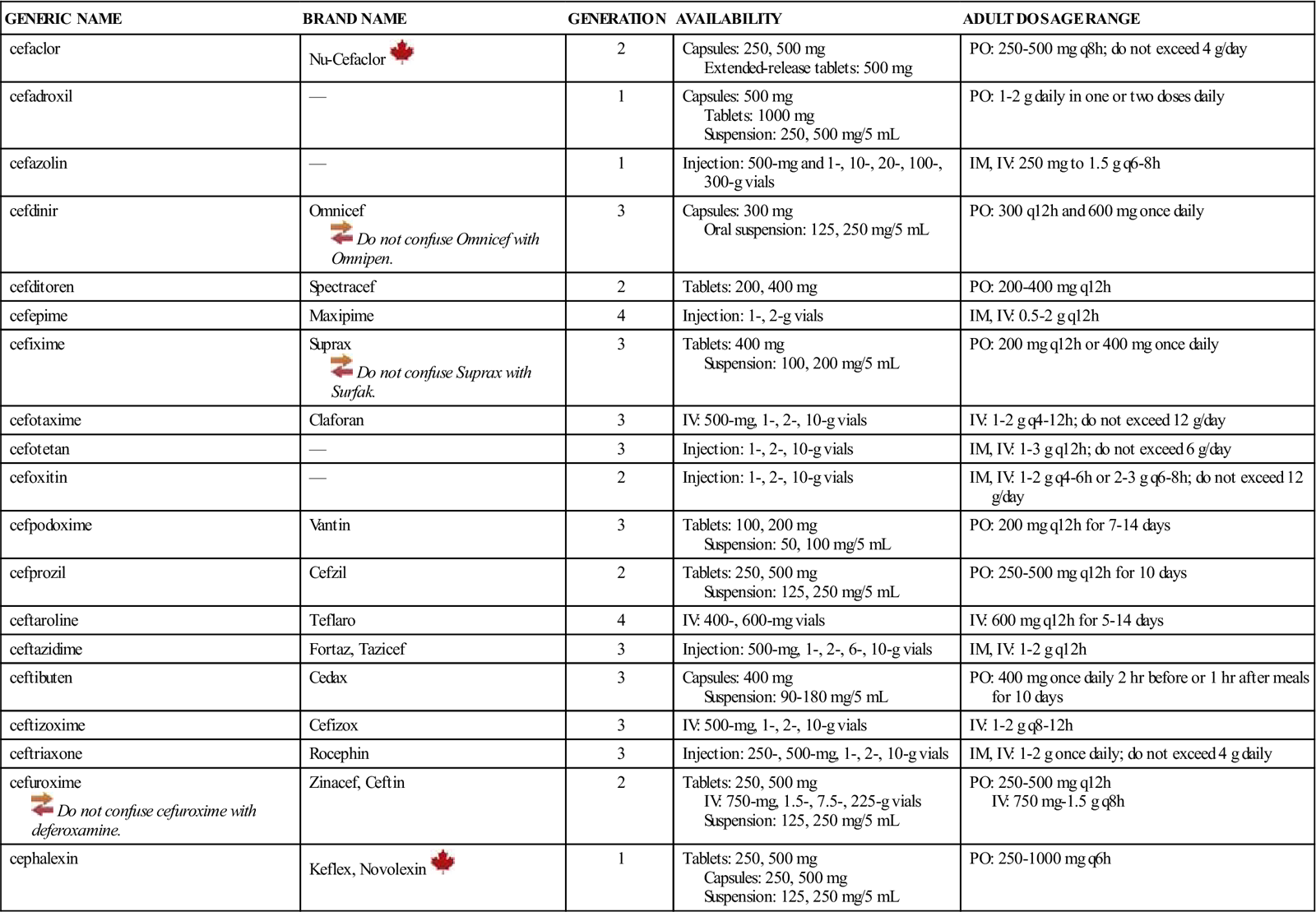
| GENERIC NAME | BRAND NAME | GENERATION | AVAILABILITY | ADULT DOSAGE RANGE |
| cefaclor | Nu-Cefaclor | 2 | Capsules: 250, 500 mg Extended-release tablets: 500 mg | PO: 250-500 mg q8h; do not exceed 4 g/day |
| cefadroxil | — | 1 | Capsules: 500 mg Tablets: 1000 mg Suspension: 250, 500 mg/5 mL | PO: 1-2 g daily in one or two doses daily |
| cefazolin | — | 1 | Injection: 500-mg and 1-, 10-, 20-, 100-, 300-g vials | IM, IV: 250 mg to 1.5 g q6-8h |
| cefdinir | Omnicef | 3 | Capsules: 300 mg Oral suspension: 125, 250 mg/5 mL | PO: 300 q12h and 600 mg once daily |
| cefditoren | Spectracef | 2 | Tablets: 200, 400 mg | PO: 200-400 mg q12h |
| cefepime | Maxipime | 4 | Injection: 1-, 2-g vials | IM, IV: 0.5-2 g q12h |
| cefixime | Suprax | 3 | Tablets: 400 mg Suspension: 100, 200 mg/5 mL | PO: 200 mg q12h or 400 mg once daily |
| cefotaxime | Claforan | 3 | IV: 500-mg, 1-, 2-, 10-g vials | IV: 1-2 g q4-12h; do not exceed 12 g/day |
| cefotetan | — | 3 | Injection: 1-, 2-, 10-g vials | IM, IV: 1-3 g q12h; do not exceed 6 g/day |
| cefoxitin | — | 2 | Injection: 1-, 2-, 10-g vials | IM, IV: 1-2 g q4-6h or 2-3 g q6-8h; do not exceed 12 g/day |
| cefpodoxime | Vantin | 3 | Tablets: 100, 200 mg Suspension: 50, 100 mg/5 mL | PO: 200 mg q12h for 7-14 days |
| cefprozil | Cefzil | 2 | Tablets: 250, 500 mg Suspension: 125, 250 mg/5 mL | PO: 250-500 mg q12h for 10 days |
| ceftaroline | Teflaro | 4 | IV: 400-, 600-mg vials | IV: 600 mg q12h for 5-14 days |
| ceftazidime | Fortaz, Tazicef | 3 | Injection: 500-mg, 1-, 2-, 6-, 10-g vials | IM, IV: 1-2 g q12h |
| ceftibuten | Cedax | 3 | Capsules: 400 mg Suspension: 90-180 mg/5 mL | PO: 400 mg once daily 2 hr before or 1 hr after meals for 10 days |
| ceftizoxime | Cefizox | 3 | IV: 500-mg, 1-, 2-, 10-g vials | IV: 1-2 g q8-12h |
| ceftriaxone | Rocephin | 3 | Injection: 250-, 500-mg, 1-, 2-, 10-g vials | IM, IV: 1-2 g once daily; do not exceed 4 g daily |
| cefuroxime | Zinacef, Ceftin | 2 | Tablets: 250, 500 mg IV: 750-mg, 1.5-, 7.5-, 225-g vials Suspension: 125, 250 mg/5 mL | PO: 250-500 mg q12h IV: 750 mg-1.5 g q8h |
| cephalexin | Keflex, Novolexin | 1 | Tablets: 250, 500 mg Capsules: 250, 500 mg Suspension: 125, 250 mg/5 mL | PO: 250-1000 mg q6h |
Monitoring
Common Adverse Effects
Gastrointestinal
Diarrhea.
Cephalosporins cause diarrhea by altering the bacterial flora of the GI tract. The diarrhea is usually not severe enough to warrant discontinuing medication. Encourage the patient not to discontinue therapy without consulting the health care provider. When diarrhea persists, monitor the patient for signs of dehydration.
Immune System (Opportunistic Infections)
Secondary Infections.
Oral thrush, genital and anal pruritus, vaginitis, and vaginal discharge may occur with cephalosporin therapy. Report promptly because these infections are resistant to the original antibiotic used. Teach the importance of meticulous oral and perineal personal hygiene.
Gastrointestinal
Hepatotoxicity.
Transient elevations of liver function test results (e.g., AST, ALT, alkaline phosphatase) have been reported. Monitor returning laboratory data and report abnormal findings to the health care provider.
Renal
Nephrotoxicity.
Transient elevations of renal test results (e.g., BUN, serum creatinine) have been reported. Renal toxicity, as evidenced by proteinuria, hematuria, casts, decreased creatinine clearance, and decreased urine output, also has been reported. Monitor returning laboratory data and report abnormal findings to the health care provider.
Hematologic
Hypoprothrombinemia.
Hypoprothrombinemia, a reduction in circulating prothrombin, with and without bleeding, has been reported. This rare occurrence is most frequent in older adult, debilitated, or otherwise compromised patients with borderline vitamin K deficiency. Treatment with broad-spectrum antibiotics eliminates enough GI flora to cause a further reduction in vitamin K synthesis.
Assess the patient for ecchymosis after minimal trauma, prolonged bleeding at an infusion site or from a surgical wound, or the development of petechiae, bleeding gums, or nosebleeds. Notify the health care provider of any of the signs of hypoprothrombinemia. The usual treatment is administration of vitamin K.
Electrolyte Imbalance.
If a patient develops hyperkalemia or hypernatremia, consider the electrolyte content of the antibiotics. Most cephalosporins have a high electrolyte content.
Vascular
Thrombophlebitis.
Phlebitis and thrombophlebitis, or inflammation of the vein with a blood clot or thrombus, are recurrent problems associated with IV administration of cephalosporins. Use small IV needles in large veins and alternate infusion sites, if possible, to minimize irritation. Carefully assess patients receiving IV cephalosporins for the development of thrombophlebitis. Inspect the area frequently when providing care; inspect during dressing changes and when the IV is changed to a new site. Always investigate pain at the IV site. Report redness, warmth, tenderness to touch, or edema in the affected part. If in the lower extremities, dorsiflexion of the foot may cause pain in the calf area (Homans’ sign). Compare findings in the affected limb with those in the unaffected limb.
Drug Interactions
Nephrotoxic Potential.
Patients receiving cephalosporins, aminoglycosides, polymyxin B, vancomycin, and loop diuretics concurrently should be assessed for signs of nephrotoxicity. Monitor urinalysis and kidney function tests for abnormal results. Report increasing BUN and creatinine levels, decreasing urine output or decreasing specific gravity (despite amount of fluid intake), casts or protein in the urine, frank blood or smoky-colored urine, or RBCs in excess of 0 to 3 RBCs/HPF (see Table 42-1) on the urinalysis report.
Antacids.
Antacids inhibit the absorption of cefaclor, cefdinir, and cefpodoxime. If antacids must be taken, the antibiotic should be taken 2 hours before or after the antacid.
Histamine-2 (H2) Antagonists.
H2 antagonists (e.g., cimetidine, famotidine, nizatidine, ranitidine) inhibit the absorption of cefpodoxime and cefuroxime, decreasing the antibiotic effect. Because of the long duration of action of the H2 antagonists, it is recommended that they not be administered when these cephalosporins are prescribed.
Iron Supplements.
Iron supplements and food fortified with iron inhibit the absorption of cefdinir. If iron supplements must be taken, the antibiotic should be taken 2 hours before or after the iron supplement.
Probenecid.
Patients receiving probenecid in combination with cephalosporins are more susceptible to toxicity because of the inhibition of excretion of the cephalosporins by probenecid. Monitor closely for adverse effects.
Alcohol.
Instruct the patient to avoid alcohol consumption during cefmetazole, cefoperazone, cefotetan, and possibly ceftizoxime therapy. Patients ingesting alcohol during, and for 24 to 72 hours after, administration of these cephalosporins will become flushed, tremulous, dyspneic, tachycardic, and hypotensive. Also, tell the patient not to use OTC preparations containing alcohol, such as mouthwash (e.g., Cēpacol, Listerine) or cough preparations.
Oral Contraceptives.
Cephalosporins may interfere with the contraceptive activity of oral contraceptives. Oral contraceptives should not be discontinued, but counseling regarding use of additional methods of contraception (e.g., condoms and foam) should be planned.
Drug Class: Glycylcyclines
Actions
Tigecycline is the first of a new family of antimicrobial agents known as the glycylcyclines. Tigecycline is chemically related to the tetracyclines but is not susceptible to the mechanisms that cause resistance to the tetracyclines. It acts by binding to the 30S ribosome, preventing protein synthesis. It is a bacteriostatic antibiotic effective against a broad spectrum of gram-positive, gram-negative, and anaerobic microorganisms. It is not effective against viruses.
Uses
Tigecycline is used to treat complicated skin and skin structure infections (cSSSIs) caused by E. coli, Enterococcus faecalis, S. aureus (methicillin-susceptible and methicillin-resistant isolates), S. agalactiae, and Bacteroides fragilis. It may also be used to treat complicated intra-abdominal infections caused by Citrobacter freundii, Enterobacter cloacae, E. coli, Klebsiella oxytoca, K. pneumoniae, E. faecalis, S. aureus (methicillin-susceptible isolates only), B. fragilis, B. vulgaris, C. perfringens, and Peptostreptococcus micros. In an effort to slow the development of strains of bacteria resistant to tigecycline, it should be used only when the pathogen is resistant to other available antibiotics.
Tigecycline is not approved for use in people younger than 18 years. As with tetracyclines, tigecycline administered during the ages of tooth development (the last half of pregnancy through 8 years of age) may cause enamel hypoplasia and permanent yellow, gray, or brown staining of the teeth.
Therapeutic Outcome
The primary therapeutic outcome expected from tigecycline therapy is elimination of bacterial infection.
 Nursing Implications for Tigecycline
Nursing Implications for Tigecycline
Premedication Assessment
Availability
IV: 50 mg in 5-mL vials.
Dosage and Administration
IV: Initial: 100 mg followed by 50 mg every 12 hours. Administer by IV infusion over 30 to 60 minutes. Therapy is continued for 5 to 14 days, depending on the severity and site of the infection and the patient’s clinical progress.
Monitoring
Common Adverse Effects
Gastrointestinal
Gastric Irritation.
The most common adverse effects of tigecycline therapy are nausea and vomiting (30% and 20%, respectively). These adverse effects are usually mild and moderate in the first 1 to 2 days of therapy but tend to resolve with continued therapy.
Severe Diarrhea.
Rarely, severe diarrhea may develop from the use of tigecycline. Report diarrhea of five or more stools daily to the health care provider. This may be an indication of drug-induced pseudomembranous colitis. Blood or mucus in the stool also should be reported to the health care provider. Warn patients not to treat diarrhea themselves when taking this drug. The use of diphenoxylate, loperamide, or paregoric may prolong or worsen the condition.
Integumentary
Photosensitivity.
Photosensitivity resulting in an exaggerated sunburn after short exposure has been reported. The patient should be cautioned to avoid exposure to sunlight and ultraviolet light. Suggest wearing long-sleeved clothing, a hat, and sunglasses when outdoors. Discourage the use of tanning lamps. Consult the health care provider about the advisability of discontinuing therapy.
Drug Interactions
Warfarin.
This medication may enhance the anticoagulant effects of warfarin. Observe for petechiae, ecchymoses, nosebleeds, bleeding gums, dark tarry stools, and bright red or coffee-ground emesis. Monitor the PT and international normalized ratio (INR), and reduce the dosage of warfarin if necessary.
Oral Contraceptives.
Tigecycline may interfere with the contraceptive activity of oral contraceptives. Oral contraceptives should not be discontinued, but counseling regarding use of additional methods of contraception (e.g., condoms and foam) should be planned.
Drug Class: Ketolides
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 )
) )
) )
) )
)