CHAPTER 36. Fluids and Electrolytes
Betty Kuiper
Water is the most abundant fluid medium in the body, composing 60% of total body weight for the average adult, 80% in a full-term infant, and as little as 45% to 55% in an older adult. 6 In a healthy physiologic state this fluid medium has a constant balance of electrolytes controlled by a unique system of checks and balances. Effects of fluid and electrolyte disturbances are often a primary or secondary reason for many emergency department (ED) visits. Fluid and electrolyte abnormalities may be caused by gastrointestinal (GI), urologic, cardiac, respiratory, and endocrine diseases and many forms of traumatic injury.
This chapter describes the interrelationship of water, water metabolism, and electrolyte composition. Fluid and electrolyte control mechanisms, signs and symptoms, etiology, and treatment of specific fluid and electrolyte abnormalities are discussed.
PATHOPHYSIOLOGY
Water and electrolytes are interdependent. The pathophysiology of one affects the function and value of the other. Normal fluid and electrolyte levels are the result of structural, physiologic, and environmental factors.
Water
Water has many important metabolic functions, including transport of nutrients and other essential substances, removal of metabolic waste products, normal cellular metabolism, and maintenance of normal body temperature. Age, weight, body fat, gender, and environmental factors such as ambient temperature determine individual fluid requirements. Fat is virtually water free; therefore increases in body fat are associated with decreases in the percentage of body water. The average adult ingests 1500 to 2000 mL of water per day. 2 In addition, the body produces 250 mL of water per day as a result of oxidation of food. 3
The most common areas of the body for fluid excretion are the bowels, skin, lungs, and kidneys. The kidneys are the primary regulators of fluid and electrolyte balance. 6 Approximately 180 L of plasma are filtered daily by the kidneys of a healthy adult. 6 From this volume, approximately 1500 mL of urine is excreted daily. 6
Total body water (TBW) is distributed between extracellular and intracellular compartments. Extracellular fluid (ECF) constitutes one third of TBW, or approximately 15 L in the average (70-kg) adult male. 6 Plasma, interstitial fluid, cerebrospinal fluid, intraocular fluid, fluids of the GI tract, and fluids of potential spaces (i.e., pleural space, peritoneal space) are examples of ECF. 6 Intracellular fluid (ICF) accounts for two thirds of TBW and represents the sum of fluid content for all the cells in the body, approximately 27 L in the average (70-kg) adult male. 6
Two regulatory mechanisms influential in maintaining normal water volume and tonicity or osmotic pressure are thirst and renal function. Thirst is the primary regulator for intake of water. It is triggered by receptors in the anterolateral hypothalamus that respond to increased plasma osmolality (as little as 2%) or decreased body fluid volume. 1 Thirst ensures adequate replacement of fluid losses and is stimulated by ECF hypertonicity and decreased ICF volume. 4 Similarly, thirst is depressed by ECF hypotonicity and increased ICF volume. Because the thirst mechanism is triggered by increased osmolality, thirst is not effective in hypotonic or hyponatremic dehydration, in which water and sodium losses are equal. Hypothalamic dysfunction also decreases the capacity for thirst. 1 Other factors that adversely affect the thirst mechanism include brain injury and psychosocial factors such as depression, confusion, and fear of incontinence.
Renal regulation of water balance is twofold, affecting both tonicity and body water. When glomerular filtrate is hypertonic, osmoreceptors in the hypothalamus are stimulated, and antidiuretic hormone (ADH) is released by the pituitary gland. ADH makes renal collecting tubules more permeable to water, so water is reabsorbed into the body, diluting blood and concentrating urine. If plasma or glomerular filtrate is hypotonic, ADH secretion is inhibited and collecting tubules reabsorb less water. Blood becomes concentrated and the urine is diluted as more water exits the kidneys.
The kidneys, through the renin-angiotensin-aldosterone system, regulate the volume of body water. When ECF volume, specifically blood volume, is low, receptors in the kidneys secrete an enzyme called renin. Renin stimulates angiotensinogen (a normal plasma protein) to release angiotensin I, which is then converted to angiotensin II by another enzyme, primarily in the lungs. Angiotensin II stimulates the adrenal cortex to secrete aldosterone, which increases sodium reabsorption from glomerular filtrate in exchange for potassium and hydrogen ions. This exchange increases plasma tonicity, which leads to ADH secretion, water retention, and increased volume. With excessive ECF volume (blood volume), aldosterone secretion is depressed, so tubular reabsorption of sodium and water decreases.
Electrolytes
An electrolyte is a substance capable of carrying an electrical charge. An electrolyte with a positive charge is called a cation, whereas an electrolyte with a negative charge is an anion. 5 Electrolytes are found in varying concentrations in ECF and ICF (Figure 36-1).
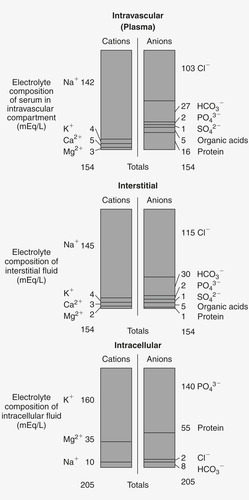 |
| FIGURE 36-1 Electrolyte content of fluid compartments. (From Lewis SL, Heitkemper MM, Dirksen SR et al: Medical-surgical nursing: assessment and management of clinical problems, ed 7, St. Louis, 2007, Mosby.) |
For the purposes of this chapter, serum electrolyte measurements are equivalent to extracellular electrolyte values (Table 36-1). Direct measurement of intracellular electrolyte concentrations in the clinical setting is not yet feasible, so ICF electrolyte concentrations must be inferred from serum electrolyte values.
| Anions | Normal Value |
|---|---|
| Bicarbonate (HCO 3−) | 22-26 mEq/L (22-26 mmol/L) |
| Chloride (Cl −) | 96-106 mEq/L (96-106 mmol/L) |
| Phosphate (PO 43−) | 2.8-4.5 mg/dL (0.90-1.45 mmol/L) |
| Cations | Normal Value |
| Potassium (K +) | 3.5-5.0 mEq/L (3.5-5.0 mmol/L) |
| Magnesium (Mg +) | 1.5-2.5 mEq/L (0.75-1.25 mmol/L) |
| Sodium (Na +) | 135-145 mEq/L (135-145 mmol/L) |
| Calcium (Ca +) (total) | 4.5-5.5 mEq/L (2.25-2.75 mmol/L) |
| Calcium (ionized) | 4.5-5.5 mg/dL (1.13-1.38 mmol/L) |
All fluids outside the cells are collectively referred to as the ECF. Electrolytes in the ECF, from greatest to least concentration, are sodium, chloride, potassium, bicarbonate, and hydrogen. ECF also contains oxygen, carbon dioxide, proteins, and a few miscellaneous anions.
ICF represents fluid found in cells in the body, about 27 L. 2 Electrolytes in the ICF, from greatest to least concentration, are potassium, phosphate, and sulphate combined; magnesium; and lastly sodium, hydrogen, and bicarbonate in equal concentrations. The ICF also contains a number of proteins.
The delicate balance of water and electrolytes between intracellular and extracellular compartments is an ongoing process of checks and balances easily disturbed by disease or injury. Regulatory processes and the role of each electrolyte are described in the following sections.
Sodium
Sodium, the principal cation in ECF, is primarily responsible for osmotic pressure. 6 Forty percent of the body’s sodium is in blood and ECF; the remainder is intracellular and in bone and connective tissue. Sodium is exchangeable across cell membranes to maintain sodium and water balance and normal arterial pressure. Sodium and chloride play an important role in maintaining body water; movement of glucose, insulin, and amino acids across cell membranes; and maintaining muscle strength, neural function, and urinary output. Sodium is essential for the sodium-potassium pump, which moves sodium and potassium across the cell membrane during repolarization (Figure 36-2). As sodium diffuses into the cell and potassium out of the cell, an active transport system supplied with energy delivers sodium back to the extracellular compartment and potassium to the intracellular compartment. 8
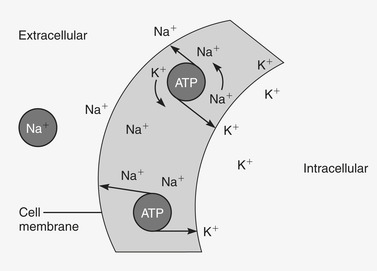 |
| FIGURE 36-2 Sodium-potassium pump. As sodium diffuses into the cell and potassium out of the cell, an active transport system supplied with energy delivers sodium back to the extracellular compartment and potassium to the intracellular compartment. ATP, Adenosine triphosphate. (From Lewis SL, Heitkemper MM, Dirksen SR et al: Medical-surgical nursing: assessment and management of clinical problems, ed 7, St. Louis, 2007, Mosby.) |
Sodium levels are maintained through the renin-angiotensin-aldosterone system, sympathetic nervous system, and a less well-defined system mediated by atrial natriuretic factor. 6 Decreased fluid volume decreases blood flow and arterial pressure, which stimulate baroreceptors in the kidneys (Figure 36-3). Baroreceptors stimulate the sympathetic nervous system, which leads to vasoconstriction of renal arterioles, decreased glomerular filtration rate, and retention of sodium and water. The opposite sequence of events occurs when fluid intake (or blood volume) rises above normal.
 |
| FIGURE 36-3 Baroreceptor response. |
Atrial natriuretic hormone (ANH), released from the atria in response to increased arterial pressure, produces natriuresis (excretion of abnormal amounts of sodium in the urine), diuresis, vasodilation, and antagonistic effects on ADH release, renin, and aldosterone. 5 The resulting increase in sodium excretion eliminates excess volume.
Chloride
Chloride, the principal anion of blood and ECF, is secreted in various body fluids along with other electrolytes. Sodium and chloride are excreted in sweat, bile, pancreatic fluids, and intestinal fluids. Gastric juice contains chloride and hydrogen. As with sodium, chloride plays a cooperative role in maintaining acid-base balance and takes part in the exchange of oxygen and carbon dioxide in red blood cells. 3 Serum chloride levels are passively regulated by serum sodium levels. When serum sodium increases, serum chloride also increases. However, chloride levels are inversely related to bicarbonate levels because chloride is sacrificed in the kidneys to produce more bicarbonate.
Potassium
Potassium is the most abundant cation in the body, with 98% in the ICF and 2% in the ECF. 3 Potassium is primarily responsible for cell membrane potential and is the counterpart to sodium in the sodium-potassium pump. Potassium governs cell osmolality and volume and is secreted in sweat, gastric juice, pancreatic juice, bile, and fluids of the small intestine.
Potassium level is primarily controlled through secretion of potassium by the distal and collecting tubules in the kidney. Potassium secretion increases in response to increased ECF potassium concentration, aldosterone levels, and distal tubular flow. A rise in ECF potassium stimulates the sodium-potassium pump located in the renal tubules. This pump maintains a low intracellular sodium concentration through exchange of potassium across the cell membrane. Increased extracellular potassium also triggers aldosterone secretion by the adrenal cortex. Aldosterone increases the rate at which tubular cells secrete potassium and the permeability of the renal tubular lumen for potassium. This is a negative feedback system regulated by the serum potassium level. Finally, increased distal tubular flow causes rapid secretion of potassium into the urine.
Alkalosis temporarily decreases serum potassium by driving potassium into the cells in exchange for hydrogen ions. 3 Conversely, acute acidosis is the major factor that decreases potassium secretion and increases serum potassium. Acute acidosis increases hydrogen ion concentration in the ECF, causing potassium to move out of the cell in exchange for excess hydrogen ions. 3
Calcium
Approximately 99% of the body’s calcium is found in bone, with 1% in ICF and 0.1% in ECF. 3 Bone acts as a large reservoir for calcium when ECF calcium levels fall. Calcium is transported in the blood in two forms. Half is bound to plasma proteins, usually albumin, and a small amount of nonionized calcium forms complexes with anions such as phosphate, citrate, and sulfate. The rest exists as an ionized form that is free and metabolically active. Most ionized calcium is found in the ECF. Because of the large amount of calcium bound to plasma proteins, assessment of total serum calcium without simultaneous measurement of serum proteins has limited value in determining hypocalcemia or hypercalcemia.
Calcium has many important functions—smooth and skeletal muscle contraction, bone and brain metabolism, blood clotting, and as a primary ingredient in lung surfactant. 5 Calcium is essential for membrane polarization and depolarization, action potential generation, neurotransmission, and muscle contraction. Calcium channels in myocardial cells allow transmembrane calcium transport.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
