CHAPTER 31. Cardiovascular Emergencies
Darleen A. Williams
The American Heart Association (AHA) estimates that in 2004 there were 15,800,000 Americans age 20 and older with some form of coronary heart disease (CHD). 3 Fifty percent of men and 64% of women who die suddenly of CHD do so without having had or recognizing any early warning signs. Using 2004 data, the AHA calculates that “every 26 seconds an American will suffer a coronary event and every minute someone will die from one.” Approximately 83% of those who die of CHD are age 65 or older; however, this is not a disease exclusive to our senior citizens. Data have shown that sudden cardiac death accounts for 19% of sudden deaths in children ages 1 to 13 and 30% between 14 and 21 years of age. 3 For many years organizations such as the AHA and the American Red Cross have worked to educate the public to become more aware of the warning signs and risk factors related to heart disease and heart attacks. The generally accepted risk factors that contribute to the development of CHD include elevated blood cholesterol levels; untreated hypertension; tobacco use; diabetes; obesity; lack of regular physical activity; poor dietary intake, including low intake of daily fruits and vegetables; and overindulgence in alcohol. In addition to the great loss of life, it is estimated by the AHA that the 2007 direct and indirect financial cost to American society was $151.6 billion.
Evidence has shown that prevention is the key to reducing complications associated with heart disease. In CHD, time really is muscle. The earlier that interventions begin, the greater the opportunity for the patient to experience a positive outcome and return to a productive life. Regulatory agencies and third-party payers are focused on ensuring that appropriate timely care is delivered. Clinical practice guidelines have been the end result of studies such as the Thrombolysis in Myocardial Infarction (TIMI-IIB), Efficacy and Safety of Subcutaneous Enoxaparin in Non–Q-Wave Coronary Events (ESSENCE), and Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS). 4
Core measures have been identified by the Centers for Medicare and Medicaid Services (CMS) to ensure that patients with acute coronary syndrome (ACS) receive appropriate evidence-based standards of care. 21 Health care facilities must meet the standards associated with the core measures or face serious sanctions and loss of financial reimbursement. Although evidence-based practice is the gold standard for care, challenges continue to arise and facilities struggle to change their policies, procedures, and practices to meet the increasing complex requirements. Data associated with core measure compliance are available for review on public access Web sites.
It is important to remember that patients affected by cardiovascular diseases do not always have obvious signs and symptoms before presenting or even during their evaluation in the emergency department (ED). Understanding basic anatomy and physiology along with disease development and its impact is imperative for those providing emergency care.
The focus of this chapter is on cardiovascular emergencies secondary to disease processes and their progression. Cardiovascular emergencies related to trauma are discussed in Chapter 23, whereas pediatric-specific cardiovascular emergencies will be covered in Chapter 47.
ANATOMY AND PHYSIOLOGY
The heart is a muscular four-chambered organ with valves that separate each chamber. The primary function of these valves is to prevent backflow of blood (Figure 31-1). Despite being a two-pump system, the heart works in synchrony. Deoxygenated blood from the venous system enters the right atrium through the inferior and superior vena cavae. Blood is then pumped from the right ventricle into the pulmonary vasculature, where it becomes oxygenated in the lungs. After the exchange of carbon dioxide and oxygen occurs, the oxygenated blood moves to the left atrium via the pulmonary veins. The left ventricle then pumps this blood, via the arterial system, to the body. Figure 31-2 illustrates blood flow through the heart. The left ventricle is stronger than the right and has the ability to pump 4 to 8 L of blood per minute. Oxygenation of the heart muscle is provided by blood from the right and left coronary arteries. The coronary arteries lie on the surface of the heart and are filled during ventricular diastole. 17
 |
| FIGURE 31-1 Anatomy of the heart. (From Lounsberry P, Frye SJ : Cardiac rhythm disorders: a nursing process approach, ed 2, St. Louis, 1992, Mosby.) |
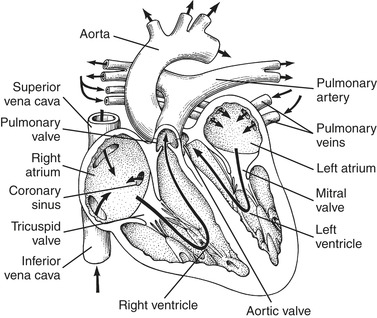 |
| FIGURE 31-2 Circulation of blood through the heart. Arrows indicate direction of flow. (From Atkinson LJ, Fortunato NM: Berry and Kohn’s operating room technique, ed 8, St. Louis, 1996, Mosby.) |
The heart is divided into three distinctive layers: epicardium, myocardium, and endocardium. The epicardium, also known as the visceral pericardium, serves as the outer layer of the heart and is where the coronary arteries lie. Next is the myocardium, the thick and muscular portion of the heart. It is composed of concentric muscular fiber rings. It is the contraction of these concentric rings that facilitates blood flow into and out of the ventricles. Finally, the endocardial layer is the innermost portion of both the atria and ventricles and is made of smooth tissue. This endocardial layer also functions as the surface for the heart valves. The entire heart is surrounded by a fibrous sac called the pericardium. This sac holds the heart in place and has fluid inside to lubricate the heart and prevent friction from occurring during contractions.
One of the most unique characteristics of cardiac tissue is its automaticity, the ability to initiate electrical activity. Figure 31-3 shows the heart’s electrical conduction system. The sinoatrial (SA) node has the highest rate of automaticity, spontaneously depolarizing between 60 and 100 times per minute. The impulses generated by the SA node are carried to the atrioventricular (AV) node by intraatrial tracts (i.e., Bachmann’s Bundle, Wenckebach’s, and Thorel’s tracts). Electrical stimulation of heart muscle begins in the atria and causes the mechanical event of atrial contraction. At the AV node there is a slight delay in the impulse transmission, which allows for atrial contraction to be completed before ventricular stimulation begins. From the AV node the electrical impulse is carried to the ventricles by the bundle of His, which includes the right and left bundle branches. The bundles terminate at the Purkinje fibers, where the impulses are delivered to the ventricular muscle, resulting in ventricular contraction.
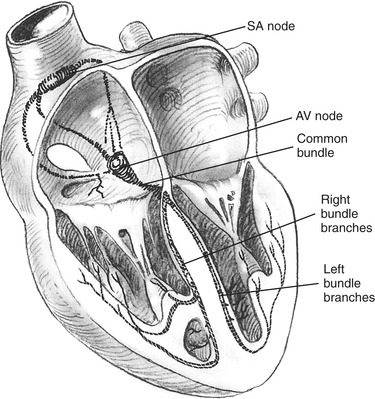 |
| FIGURE 31-3 Conduction system of the heart. AV, Atrioventricular; SA, sinoatrial. (From Davis JH, Drucker WR et al: Clinical surgery, vol 1, St. Louis, 1987, Mosby.) |
The mechanical events of the cardiac cycle are called diastole and systole. Approximately 60% of the cardiac cycle is diastole, the time when the ventricles are filling. During diastole the aortic and pulmonic valves close while the mitral and tricuspid valves open. Electrically this corresponds to electrical stimulation and mechanical contraction of the atria. As the atria contract, the pressure in the atria becomes greater than in the ventricles, causing the AV valves to open and allowing blood to flow from an area of greater pressure to an area of lower pressure (Figure 31-4). The systolic phase of the cardiac cycle corresponds with ventricular contraction and opening of pulmonic and aortic valves. During contraction, AV valves close and chordae tendineae contract to prevent any regurgitation of blood. Figure 31-5 depicts the relationship between electrical and mechanical components of the cardiac cycle.
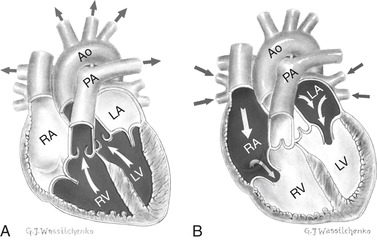 |
| FIGURE 31-4 Blood flow during (A) systole and (B) diastole. Ao, Aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle. (From Canobbio MM: Mosby’s clinical nursing series, cardiovascular disorders, vol 1, St. Louis, 1990, Mosby.) |
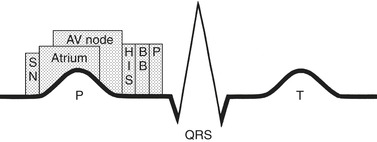 |
| FIGURE 31-5 Schematic drawing of cardiac activation related to the surface electrocardiogram (ECG). The timing of activation of the components of the conduction system is superimposed on the surface ECG. AV, Atrioventricular; BB, bundle branches; HIS, common bundle of His; P, Purkinje network; SN, sinus node. (From Lounsberry P, Frye SJ: Cardiac rhythm disorders; a nursing process approach, ed 2, St. Louis, 1992, Mosby.) |
The pressures within the cardiovascular system are affected by both preload and afterload and greatly affect cardiac output. Preload refers to the volume of blood entering the right side of the heart. Afterload refers to pressure in the arterial system that the heart must overcome to pump out its ventricular blood volume.
Cardiac activity is regulated by branches of the autonomic nervous system, and the specific effects of each branch are described in Table 31-1. Receptors in the heart and great vessels respond to signals from the sympathetic nervous system (Table 31-2). Their stimulation affects heart rate, contractility, automaticity, conduction, and vascular smooth muscle. These receptors help prepare the body for the fight-or-flight response to either perceived threats or actual physiologic changes, including blood volume loss.
| AV, Atrioventricular; SA, sinoatrial. | ||
| Nerve Activation | Cardiac Effect | Clinical Manifestations |
|---|---|---|
| Parasympathetic | Slows SA node discharge Slows AV node conduction and increases refractoriness | Symptomatic bradycardia Transient heart block |
| Sympathetic | Heart rate increases Enhances AV node function Shortens His-Purkinje and ventricular muscle refractoriness Increased ventricular contraction Increased peripheral vascular resistance | Tachycardia Hypertension Increased cardiac output |
| Sympathetic Receptor | Location | Clinical Response |
|---|---|---|
| α | Vascular smooth muscle | Vasoconstriction |
| β1 | Myocardium | Increased heart rate, contraction, automaticity, and conduction |
| β2 | Peripheral vasculature and lungs | Vasodilation of peripheral vasculature and bronchodilation |
| Dopaminergic | Renal, mesenteric, cerebral, and coronary arteries | Vasodilation |
SPECIFIC CARDIOVASCULAR EMERGENCIES
The following specific emergencies will be discussed: cardiac arrest, ACS, dysrhythmias, heart failure (HF), pericarditis, aortic aneurysm, and hypertensive crisis.
Cardiac Arrest
Sudden cardiac arrest is defined as death from a sudden loss of heart function. 23 More than 700,000 American adults die annually from cardiovascular disease; of these, approximately 850 die every day from sudden cardiac arrest. 2 A common cause of sudden cardiac arrest in adults is ventricular tachycardia (VT) that, if left untreated, deteriorates into ventricular fibrillation (VF). These lethal dysrhythmias are usually the end result of an evolving myocardial infarction; however, these dysrhythmias and resulting cardiac arrest may also be associated with aneurysm rupture, cardiomyopathies, rheumatic heart disease, mitral valve prolapse, and cardiac surgery. Sudden cardiac arrest can also be associated with many other conditions or events. Table 31-3 reviews some of the other potential causes of cardiopulmonary arrest, their causes, signs, symptoms, and therapeutic interventions. In addition, the health care team should gather information related to the events of the arrest. Determining the cause of the cardiac arrest event may assist the health care team in preventing a reoccurrence.
| COPD, Chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; ECG, electrocardiogram; IM, intramuscular; IV, intravenous; IVP, intravenous push; PEA, pulseless electrical activity; PVC, premature ventricular contraction. | ||||
| Special note: Accurate, rapid assessment and interventions are the patient’s best chance for having a good outcome. | ||||
| ∗There are many causes of cardiopulmonary arrest other than primary cardiac abnormalities, and it is for this reason that the health care provider must be very familiar with these potential causes and their associated signs and symptoms. Timely accurate identification of the cause of the patient’s problem via the use of diagnostic testing in conjunction with assessment findings will determine the definitive therapeutic interventions needed. This table lists some of these nonprimary cardiac abnormalities that may result in cardiopulmonary arrest. Also listed in the table are therapeutic interventions for each condition that in addition to basic and advanced cardiac life support measures may be necessary with these patients. | ||||
| Causes | Specific Cause | Signs and Symptoms | Therapeutic Intervention | Notes |
|---|---|---|---|---|
| Metabolic | Hypoglycemia | Loss of consciousness, Physical signs of insulin or oral hypoglycemic agent usage; tachydysrhythmias; seizures; aspiration | Dextrose, 50% IVP or if unable to obtain an IV give glucagon IM | Consider hypoglycemia a strong possibility in patients who have a history of diabetes |
| Hyperkalemia | ECG: Prolonged QT interval; peaked T waves; loss of P waves; wide QRS complexes | IV calcium chloride or calcium gluconate, sodium bicarbonate, insulin, and glucose | Often seen in hemodialysis and renal failure patients; also seen in patients taking potassium-sparing diuretics and patients with rhabdomyolysis | |
| Drug-induced | Tricyclic antidepressants, amitriptyline (Elavil), amitriptyline and perphenazine (Etrafon, Triavil), imipramine (Tofranil), doxepin (Sinequan), protriptyline (Vivactil) | TachydysrhythmiasProlonged QT/torsade de pointes | Sodium bicarbonate IV | Causes direct cardiac toxicity; often delayed toxicity in adults |
| Opiates | Bradydysrhythmias; heart blocks | Naloxone (Narcan) IV | Street drugs may be mixed with multiple substances | |
| β-Blockers | Cardiac: Heart blocks; bradydysrhythmias; PVCs | Atropine | PVCs may be caused by slow rate | |
| Respiratory: Bronchospasm | Aminophylline | |||
| Pulmonary (any disease causing severe hypoxia) | Asthma | Severe bronchospasm causing hypoxia and respiratory acidosis ECG: Tachydysrhythmias (especially ventricular fibrillation) | Endotracheal intubation and ventilatory support | Abuse of sympathomimetic inhalants |
| Pulmonary embolus | Pleuritic chest pain; shortness of breath in high-risk patients (postoperative, those taking birth control pills); syncope (recent study shows 60% have syncope as part of initial complaint); tachydysrhythmias | Good ventilatory support; consider fibrinolytic agents | Pathophysiology; acute hypoxia and cor pulmonale leading to tachydysrhythmias | |
| Tension pneumothorax | Distended neck veins; tracheal deviation; asymmetric chest expansion ECG: Often PEA | Needle thoracostomy; chest tube | Often seen in patients with blunt chest trauma; often occurs during CPR because of chest compressions (especially in patients with COPD) | |
| Neurogenic | Increased intracranial pressure from any cause (e.g., subarachnoid hemorrhage, subdural hematoma) | Central neurogenic breathing; dilated pupil(s); abnormal posturing (decerebrate/decorticate) ECG: Wide range of dysrhythmias, especially heart blocks | Central neurogenic hyperventilation (causes respiratory alkalosis, which results in cerebral vasoconstriction); steroids; diuretic agents; surgery | Damage to brainstem and autonomic centers |
| Hypovolemic | Anything that causes volume loss such as gastrointestinal bleeding, severe trauma with organ damage, ruptured ectopic pregnancy, dissecting or leaking aneurysm | Tachycardia; decreasing blood pressure; skin cool, clammy, pale; obvious signs of external blood loss | IV fluids; shock; position; surgery | A major cause of cardiopulmonary arrest that may be unrecognized |
| Other cardiac causes | Pericardial tamponade | Distended neck veins; decreasing blood pressure; distant heart sounds; widening pulse pressure ECG: PEA; bradydysrhythmias | IV fluids: Atropine; isoproterenol; pericardiocentesis; thoracotomy | Look for this especially in patients with blunt chest trauma or prolonged CPR |
In a cardiac arrest situation immediate interventions begin with initiation of basic life support. Effective cardiopulmonary resuscitation is essential for a positive outcome to occur. Advanced life support measures should not be initiated until the basics have been addressed. The steps involved in both basic and advanced life support evolve based on ongoing research; therefore all health care providers must stay abreast of the changes in order to provide patients with the best evidence-based care possible.
Basic life support begins with the primary survey and appropriate interventions. Initial measures begin with establishing an airway (remember to use techniques that also stabilize the cervical spine if mechanisms suggest a potential injury). Airway management and oxygenation are discussed in greater detail in Chapter 30. Once the airway has been established, evaluate the patient’s breathing status and provide artificial ventilations as indicated. While providing ventilations, it is important for the rescuer to ensure that the rate and volume are sufficient to oxygenate the patient. Once breathing has been ensured, chest compressions should be started, if appropriate. When performed properly, chest compressions provide and perhaps even restore cardiac circulation. Properly performed chest compressions produce approximately 30% of normal cardiac output, which is enough blood flow through the heart and brain to sustain tissue viability for a short time. Cerebral blood flow must be at least 50% of normal volume to maintain consciousness. 1 Chest compressions are best accomplished with the pulseless patient supine on a firm surface. This positioning allows for even compression of the chest cavity. Compressions should be smooth, even, and strong enough to generate a central pulse—either carotid or femoral. Defibrillation is the last step in the initial resuscitation effort. Evidence has clearly shown the need for early defibrillation in an effort to restore a viable heart rhythm. The use of electricity in resuscitation, both defibrillation and synchronous cardioversion, will be discussed in more detail later in this chapter.
Mechanical cardiopulmonary resuscitation devices have been available for years. There are many versions available; some devices simply provide chest compressions, whereas others are capable of providing both chest compressions and synchronous ventilations for the patient. There are devices available that will record the resuscitation activities, including the patient’s rhythm, compressions being performed, and any attempts at defibrillation or cardioversion provided. Advantages of using such a device are that it can assist in providing consistent chest compressions and reduce or prevent rescuer fatigue during long resuscitation events or extended transport times for rural emergency medical services units. In addition, in the absence of multiple staff to participate in the resuscitation, using a chest compression device may allow the rescuer to begin providing advanced life support measures while the basics are being done using the mechanical device. Use of these devices should be restricted to adult patients, and they should be used by specially trained and experienced personnel only. Some current animal studies have shown promise in improving the hemodynamic status of the cardiac arrest victim when a mechanical compression device was used. 17
Indications for an open thoracotomy and cardiac massage in the ED are limited to patients who are in full cardiopulmonary arrest secondary to penetrating chest trauma. Generally, patients who have sustained blunt trauma have very poor outcomes following resuscitative efforts that include an open thoracotomy. Open thoracotomy and cardiac massage are rarely performed and should be attempted only when the facility has the appropriate resources available to manage the patient if a pulse returns after open thoracotomy is performed.
Family Presence During Resuscitation
Allowing a family member to be present during resuscitation is not only a common occurrence, it is supported by many organizations, including the Emergency Nurses Association and the American Association of Critical Care Nurses. Providing the option for family presence during resuscitation is discussed in detail in Chapter 13.
Therapeutic Electrical Interventions
The use of electricity in the treatment of patients with heart disease is a common therapeutic intervention. Whether it is pacing, defibrillation, or cardioversion, electricity is frequently the lifesaving intervention for this patient population. The goal is to restore normal electrical conduction within the heart, which in turn should initiate contractions and restore essential cardiac output. Patients who are in cardiopulmonary arrest require a prompt and accurate assessment to determine their current cardiac rhythm followed by implementation of the appropriate evidence-based algorithm and emergency care. Health care providers should be aware of basic defibrillator safety concepts to avoid injury to both the patient and staff. Some noteworthy safety points to be aware of before any therapeutic electrical intervention use include the following:
• Remove all medication patches; if left on they can cause arcing during shock delivery and burn the patient.
• When paddles are used with conductive gel, ensure that just enough gel is placed on each paddle to lightly cover its surface. Excess conductive medium can run across the patient’s chest and potentially cause arcing during shock delivery, resulting in burns to both the patient and the rescuer.
• Maintain firm contact between paddles and the chest wall to deliver the desired energy and prevent arcing.
• Do not place patches/paddles directly over implanted devices.
• When hands-free adhesive pads/patches are being used, carefully inspect wires regularly for any fraying or cracking.
Always loudly announce “I’m clear, everybody clear” before delivering each shock, and physically look to ensure that everyone is clear of the patient.
DEFIBRILLATION
Defibrillation has been defined as “the arrest of fibrillation of the cardiac muscle (atrial or ventricular) with restoration of the normal rhythm if successful.”1 For practical purposes defibrillation is a definitive way in which the rescuer uses electricity in an attempt to convert a patient’s lethal rhythm into a viable one. Although exact statistics are not available, the AHA estimates that with early defibrillation (within 5 to 7 minutes) only 30% to 45% of cardiac arrest patients will survive the event, but without it 95% will die before reaching the hospital. 2
In addition to being able to recognize dysrhythmias, the emergency nurse must be knowledgeable of current evidence-based interventions for each abnormal rhythm. The first step is to be familiar with the equipment used at your facility. Is the defibrillator monophasic or biphasic? Does it function as hands-off only or paddles only? Can it pace, defibrillate, and synchronize cardiovert, or is it only an automated external defibrillator (AED)? Before using the defibrillator it is important to be sure that the machine either is plugged in or has a fully charged battery. This maintenance procedure should be performed daily to ensure that the defibrillator will be fully functional in the event of an emergency.
Previously the standard monophasic defibrillators allowed electrical current to flow in only one direction. With the advancement of technology, biphasic defibrillators have evolved, which allow the energy to flow in both directions. With biphasic energy delivery the amount of energy required to convert a lethal rhythm is significantly reduced. When deciding on the energy needed for defibrillation or synchronized cardioversion, confirm if the defibrillator is monophasic or biphasic and be aware of the manufacturer’s recommendations for energy delivery.
After confirming that the patient’s rhythm requires defibrillation, place the adhesive electrode pads (if the hands-off device is being used) on the patient’s bare chest. One pad goes to the upper chest just right of the sternum and the other at the apex of the heart on the patient’s left lateral chest, under the left breast. If paddles are used, they are held firmly in the same locations after being covered with conduction gel or placed on commercially prepared defibrillator pads (Figure 31-6).
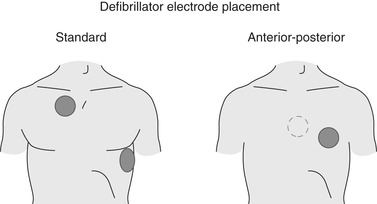 |
| FIGURE 31-6 Standard and anterior-posterior electrode placement for defibrillation. (From Rosen R, Barkin R: Emergency medicine: concepts and clinical practice, ed 4, St. Louis, 1998, Mosby.) |
Anterior/posterior placement may also be used with the hands-free method and is indicated when repeated or long-term placement may be necessary. If the patient has any implanted devices, pad/paddle placement may need to be slightly altered. Do not place the pad/paddle directly over the implanted device, and external devices should be at least 1 inch away. To deliver the defibrillation the machine must be turned on and the desired mode selected. It is important to be sure that the “defib” mode is engaged. Next the energy level must be selected/programmed in and the machine charged. When fully charged the machine will sound an alert. It is at this time that the rescuer will loudly announce that a shock is going to be delivered, “I’m clear, everybody clear,” and physically look to verify that all personnel are free from contact with the patient. To deliver the energy via the pads, press and hold the appropriate button on the device until the shock is delivered; with the paddles, press the discharge buttons on the paddle handles simultaneously. In the defibrillation mode the shock will be delivered immediately; rescuers should resume chest compressions immediately after the shock is delivered.
Another electrical delivery device available is the AED. There are many versions of this self-contained defibrillator available, and it is an integral part of the public access defibrillation program. Some health care facilities have chosen to place these lifesaving devices throughout their buildings and on low-acuity units where need is infrequent and staff are not trained in advanced resuscitation skills. The vast majority of AEDs use biphasic technology; some are fully automated with no oscilloscope, whereas others are semiautomated and require the user to have rhythm-recognition skills. Both types of AED use only “hands-free” defibrillation. Once properly placed on the patient, the device will analyze the rhythm and decide if a shock is needed. The fully automated AED will deliver the shock automatically, whereas the semiautomated AED requires the rescuer to follow the audible instructions step-by-step to deliver the shock. Introduction and training for AEDs are done in basic life support classes. In an effort to assist people with remembering the steps, the PAAS mnemonic was developed: power, attach, analyze, shock. As with all electrical devices, use the safety concepts discussed earlier.
Successful defibrillation depends on multiple factors. The reason the patient arrested is important. Determining the cause will be instrumental in correcting the issue and preventing reoccurrence. Using the AHA advanced cardiac life support (ACLS) guidelines, health care providers should consider reasons for the initial arrest (Table 31-4) and perhaps identify the reason for an unsuccessful defibrillation.
| H’s | T’s |
|---|---|
| Hypovolemia (bleeding or dehydration) | Thrombosis (coronary or pulmonary) |
| Hypoxia | Tension pneumothorax |
| Hypokalemia/hyperkalemia | Tamponade (cardiac)Tablets (overdose or ingestion) |
| Hypothermia | Toxins |
| Hydrogen ion (acidosis) | Trauma |
| Hypoglycemia |
CARDIOVERSION
Synchronized cardioversion is used when either the patient has become hemodynamically unstable or pharmacologic interventions have been unsuccessful in managing sustained ventricular tachycardia, supraventricular tachycardia, atrial fibrillation, or atrial flutter. In synchronized cardioversion, energy is timed with the QRS complex and avoids shock delivery during the relative refractory period, which could produce ventricular fibrillation. Synchronized cardioversion decreases potential energy delivery during the vulnerable period of repolarization, that is, the T wave of the electrocardiogram (ECG) (Figure 31-7).
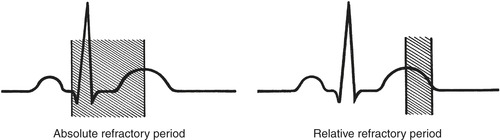 |
| FIGURE 31-7 Absolute and relative refractory periods on the electrocardiogram. |
The procedure for cardioversion is the same as for defibrillation with three important distinctions: (1) the machine must be set on synchronous mode; (2) sedation should be given for the conscious patient if time allows; and (3) when the delivery button is pushed, there will be a slight delay in firing because the machine is sensing the R wave in order to deliver the energy at the precise moment. Anxiolytics such as diazepam (Valium) or midazolam (Versed) should be administered intravenously (IV) in small, incremental doses before energy delivery. The procedure should be explained to the patient and informed consent obtained whenever possible.
As has been previously discussed, it is important to determine the cause of the patient’s current condition. Potential treatable causes of cardiac arrest are found in Table 31-4. When possible a baseline 12-lead ECG should be obtained before and after the attempted cardioversion. Immediately after the procedure the patient’s cardiac rhythm, vital signs, and level of consciousness should be assessed and closely monitored until the patient is hemodynamically stable and returns to an acceptable rhythm. Complications of cardioversion include asystole, junctional rhythms, premature ventricular contractions (PVCs), ventricular tachycardia, ventricular fibrillation, embolization, and return to the original dysrhythmia.
Another method to convert a patient’s rhythm is through manual stimulation of the vagus nerve using a Valsalva maneuver such as causing the patient to gag or vomit. Stimulation of the vagus nerve will slow the heart rate and may terminate the dysrhythmia. In the past, ocular pressure and application of ice water to the patient’s face have been used; however, these methods are no longer recommended.
The physician may try carotid sinus massage to convert the rhythm. This is accomplished by placing pressure on the carotid bodies, which stimulates the baroreceptors and therefore the parasympathetic branch of the autonomic nervous system. This stimulation decreases blood pressure and heart rate. Only one side should be done at a time. Before attempting this procedure the physician should auscultate the carotid arteries for bruits. Bruits are produced by turbulent flow through the carotid arteries and are suggestive of atherosclerotic plaque in the artery. If any of this plaque were dislodged during the procedure, the patient would be at risk for a stroke. If a bruit is heard, carotid massage is contraindicated. If no bruits are heard and the procedure will be attempted, the patient should be supine on supplemental oxygen and have at least one patent IV access. Before, during, and after the attempt the patient should be closely monitored. In more than 75% of the population, preferential massage of the right carotid body affects the SA node, whereas left carotid body massage affects the AV node. Even when the SA node is completely shut down, the AV node can provide pacemaker activity. If the left side is massaged first, complete block of the AV node could occur and lead to a slow ventricular rate. Pressure must be applied gently to the carotid artery just below the mandible and in small, circular motions, rotating the fingers backward and medially. Carotid massage should not exceed 5 to 10 seconds and should be discontinued sooner if the rhythm changes. Even when properly performed, carotid massage may cause asystole for 15 to 30 seconds, followed by a few idioventricular complexes before a new pacemaker site becomes active. Emergency resuscitation equipment and medications should be readily available whenever carotid massage is performed. If carotid massage is successful, continue to monitor the patient for several hours. Complications of carotid massage include further dysrhythmias (e.g., ventricular tachycardia, ventricular fibrillation, asystole), stroke, cerebral anoxia, and seizures. 1
PACEMAKERS
A pacemaker is an electrical device that may restore an adequate heart rate and cardiac output. It can be used either via the transvenous or transcutaneous routes. A transcutaneous pacemaker (TCP) is a temporary intervention for patients experiencing symptomatic, unstable bradycardia, and second- or third-degree heart blocks because it is easily applied and managed until the patient can receive definitive treatment. The TCP can be applied by following these steps: (1) place the pacing electrodes on the patient’s bare chest as recommended by the manufacturer; (2) turn the pacemaker on, and set the demand rate as ordered by the physician (usually between 60 and 70 beats/min); and (3) turn up the milliamperes (mA) current slowly, increasing the dose until capture occurs with pacer spikes noted on the patient’s monitored rhythm. Keep the pacer’s mA at the lowest level possible that still maintains electrical capture (pacer spikes) and mechanical capture (patient’s pulse) and at a rate that keeps the patient clinically stable. Continuous pacing can be uncomfortable; therefore analgesia or anxiolytics should be considered.
Patients with complete heart block may be unable to electrically or mechanically respond to the pacemaker stimulus. Successful pacing depends on the condition of the myocardium. Mechanical capture is evaluated by the presence of a pulse consistent with paced beats. Both types of capture (electrical and mechanical) must be present for pacing to be considered effective. Two major reasons for lack of capture are acidosis and hypoxemia. Evaluate the patient’s oxygen saturation and acid-base levels to determine if further airway or ventilatory interventions are needed. If there is a lack of pacer spikes, begin by checking the TCP electrodes followed by inspecting all the wires and connections. If necessary replace electrodes to ensure contact between external pacing electrodes and skin surface is adequate. Skin should be clean and dry before electrode application. Tincture of benzoin may be used to improve adherence to the skin if the patient has been diaphoretic.
IMPLANTABLE CARDIOVERTER-DEFIBRILLATOR
The implantable cardioverter-defibrillator (ICD) is a small generator that is used in patients at risk for life-threatening dysrhythmias—especially ventricular dysrhythmias. The ICD device is surgically placed under the skin in the chest wall just below the clavicle or in the abdominal cavity. This minigenerator monitors the patient’s rhythm, providing pacing, cardioversion, or defibrillation based on the patient’s needs, device capability, and programming.
When a patient with an ICD requires external defibrillation because the implanted device has malfunctioned or failed, it is important to know how to perform this intervention in a safe and effective manner. Defibrillator paddles or hands-free adhesive patches must not be placed directly over the ICD device. If standard paddle/patch placement and defibrillation attempts are unsuccessful, the resuscitation team should try the anterior-posterior placement for defibrillation. Health care professionals who have direct physical contact with the patient may experience a slight harmless tingling sensation when the ICD device fires. If the ICD fires inappropriately, the device can be deactivated by placing a magnet over the ICD generator. 1
Resuscitation Interventions
FLUID RESUSCITATION
The use of IV fluids during resuscitation must be determined on an individual patient basis. Some patients may need crystalloid fluid boluses secondary to volume loss, whereas others may be in a fluid overload state and need to have intake strictly limited. In addition to the patient’s current status, it is important to gather as much of the health history as possible because this information will influence the amount, rate, and type of IV fluids administered. After every intervention it is necessary to reassess and evaluate the patient’s response. Because of adverse effects on cerebral tissue, dextrose-containing solutions are not recommended during resuscitation; instead the fluids of choice are normal saline or lactated Ringer’s solution.
PHARMACOLOGIC THERAPY
Emergency drug therapy depends on the patient’s cardiac rhythm, 12-lead ECG, and hemodynamic status. Table 31-5 provides an overview of commonly used emergency medications. The first-line drug in management of cardiac arrest, specifically asystole, pulseless electrical activity (PEA), VF, and pulseless VT is either epinephrine or vasopressin. 1 It is important to remember that the management of VF and pulseless VT requires immediate defibrillation followed by chest compressions before the administration of medications.
| AV, Atrioventricular; D 5W, 5% dextrose in water; gtt, drops, IO; intraosseous; IV, intravenous; NS, normal saline; PAC, premature atrial contraction; PSVT, paroxysmal supraventricular tachycardia; PVC, premature ventricular contraction; RSI, rapid sequence intubation; SA, sinoatrial; SVT, supraventricular tachycardia; VF, ventricular fibrillation; VT, ventricular tachycardia. | |||||
| Drug | Category | Actions | Indications | Dose | Comments |
|---|---|---|---|---|---|
| Adenosine (Adenocard) | Unclassified antidysrhythmic | Slows conduction through the AV node; also can interrupt the reentry pathways through the AV node to decrease the heart rate | PSVT | PSVT: 6 mg IV/IO rapid push over 1-3 seconds; may give an additional 12 mg IV/IO rapid push if first dose is not effective; may repeat a second 12 mg IV/IO rapid push after 1-2 min It is helpful to administer 20 mL of normal saline by rapid bolus after giving adenosine to clear the IV tubing | May cause brief heart block or transient asystole; may cause other dysrhythmias (e.g., PVC, PAC, sinus bradycardia, sinus tachycardia) during time of conversion from PSVT These symptoms are usually brief because of the short half-life of the drug (i.e., <10 seconds) Contraindications include second- or third-degree AV block or sick sinus syndrome |
| Amiodarone (Cordarone) | Combined β-adrenergic blocker and calcium channel blocker | Prolongs myocardial cell action potential duration and refractory period; decreases AV conduction and sinus node function | Recurring ventricular fibrillation and unstable ventricular tachycardia | Cardiac arrest: 300 mg IV/IO push diluted in 20-30 mL of D 5W If no response in 3-5 min may be followed by 150 mg IV/IO push Nonarrest: Initial infusion: 150 mg over 10 min; can repeat every 10-15 min as needed Early maintenance: 1 mg/min for 6 hours Late maintenance: 0.5 mg/min Do not exceed 2.2 g in 24 hours, including initial dose | May cause hypotension, nausea, bradycardia |
| Atropine sulfate | Parasympatholytic; anticholinergic | Increases the rate of SA node firing; increases conduction through AV node; decreases vagal tone | Hemodynamically significant bradycardia; asystole; high-degree AV blocks | Bradycardia: 0.5-1 mg IV every 3-5 min max dose 0.04 mg/kg Asystole/PEA: 1 mg IV/IO push repeat every 3-5 min up to a total of 3 mg ACS: 0.06-1 mg IV repeated every 5 min to max 0.04 mg/kg Endotracheal: 2-3 mg diluted in 10 mL water or NS | In symptomatic patients do not delay pacing to administer medication. May cause paradoxic slowing of heart rate when given slowly or in doses of less than 0.5 mg |
| Epinephrine (Adrenalin) | Sympathomimetic | Both α- and β-adrenergic effects; increases mean arterial pressure; decreases fibrillatory threshold; stimulates heart in asystole and idioventricular rhythms | Allergic reaction; cardiac arrest; bronchoconstriction or bronchospasm | Cardiac arrest: 1 mg IV/IO push; or endotracheally at 2-2.5 mg, repeat either dose every 5 min when needed and follow with 20 mL NS flush | Available as 1:10,000 solution (1 mg in 10 mL) May cause tachycardia, palpitations, PVCs, angina |
| Isoproterenol (Isuprel) | Sympathomimetic | Nonspecific β-adrenergic stimulation | Hemodynamically significant bradycardia refractory to atropine Refractory torsades de pointes Temporary treatment of bradycardia in heart transplant patients | Bradycardia: 1 mg in 250 mL D 5W (4 mcg/mL); 2-10 mcg/min IV titrate to achieve desired heart rate | Causes an increased workload on the heart; use with extreme caution: exacerbates ischemia and extends infarct |
| Lidocaine (Xylocaine) | Category IB antidysrhythmic | Decreases automaticity; suppresses ventricular ectopy; depresses conduction through reentry pathways; elevates VF threshold | PVCs, VT, VF, preintubation for patients with suspected increased intracranial pressure or laryngospasm | Pulseless VF and VT: 1-1.5 mg/kg IV/IO push; may repeat in 3-5 min; do not exceed 3 mg/kg; endotracheal 2-4 mg/kg PVCs and VT: 0.5-0.75 mg/kg IV push up to 1-1.5 mg/kg every 5-10 min; maximum total not to exceed 3 mg/kg IV infusion: 1 g in 250 mL D 5W (4 mg/mL) at 2-4 mg/min (30-60 μgtt/min) RSI preintubation: 1.5-2 mg/kg IV over 30-60 seconds, wait 90 seconds then intubate | May cause central nervous system depression, drowsiness, dizziness, confusion, and anxiety Contraindications include bradycardia and related PVCs, idioventricular rhythm; if given too rapidly, may cause seizures |
| Procainamide (Pronestyl) | Category IA antidysrhythmic | Suppresses PVCs; suppresses reentry dysrhythmias; may elevate VF threshold; negative chronotrope and dromotrope; mild negative inotrope; potent peripheral vasodilator | PVCs and VT refractory to lidocaine; hemodynamically significant SVT | IV push: 100 mg slow IV push 20 mg/min; may repeat every 5 min; dose not to exceed 17 mg/kg IV infusion: 1 g in 250 mL D 5W (4 mg/mL) at 1-4 mg/min (15-60 μgtt/min) | May cause hypotension, bradycardia Contraindications: Third-degree AV block, digoxin toxicity |
| Sodium bicarbonate | Alkalotic agent | Buffers or neutralizes metabolic acidosis | Suspected acidosis in cases of cardiac arrest | 1 mEq/kg IV push; repeat 0.5 mEq/kg every 10 to 15 min when required | May inactivate catecholamines when given together in the same IV line; when possible use arterial blood gas levels to guide administration |
| Vasopressin | An alternative to epinephrine | 40 units IV/IO push May be given via endotracheal tube but no evidence-based dose recommended at this time | |||
| Verapamil (Calan, Isoptin) | Category IV antidysrhythmic | Blocks entry of Ca 2+ into cells; negative dromotrope and depresses atrial automaticity; negative chronotrope; negative inotrope; vasodilator | SVT | 2.5-5 mg IV over 2 min Over 3 min in older adults; Second dose 5-10 mg if needed Maximum dose 20 mg | May cause hypotension |
Patients with ventricular dysrhythmias associated with cardiopulmonary arrest may benefit from amiodarone (Cordarone) administration. Other antidysrhythmic agents considered for management of patients with VF or pulseless VT include lidocaine (Xylocaine), magnesium, and procainamide (Pronestyl). After the ventricular dysrhythmia is controlled, an infusion of the converting agent is necessary to maintain therapeutic drug levels. Antidysrhythmics should not be given to patients with third-degree AV block, with an escape rhythm, or with bradycardia and PVCs. Ectopic beats may contribute to the patient’s cardiac output; therefore lidocaine could effectively reduce output and cause further decompensation or asystole.
Bradycardic dysrhythmias are initially managed with atropine, which blocks stimulation of the vagus nerve. Atropine may also be used for asystole and PEA. Atropine may not be effective for high-degree AV block dysrhythmias. Isoproterenol (Isuprel) is a β-adrenergic agonist and may be used to increase cardiac output in bradydysrhythmias. Routine use of isoproterenol is not recommended because of effects on ventricular irritability; however, it is considered a first-line drug for the heart transplant patient with symptomatic bradycardia. Atropine is not used for heart transplant patients because the vagus nerve is not reattached during the transplant. Bradycardic rhythms that affect hemodynamic stability may ultimately require cardiac pacing.
Supraventricular rhythms impair effective cardiac output by decreasing cardiac filling time and may decrease the patient’s hemodynamic stability. Adenosine (Adenocard) is used to treat reentry dysrhythmias. It is an extremely fast-acting drug with a half-life of less than 10 seconds. For this reason it must be given rapidly and in a proximal vein, then followed with a 20-mL saline bolus. Side effects include flushing, dyspnea, hypotension, chest pain, transient bradycardia, transient asystole, and ventricular ectopy. These side effects will usually terminate spontaneously without further medical or nursing interventions. Other pharmacologic agents such as ibutilide, β-adrenergic blocking drugs (e.g., metoprolol [Lopressor]), and calcium channel blocking agents (e.g., verapamil [Calan] and diltiazem [Cardizem]) can be used for controlling ventricular response rate in patients with supraventricular tachycardias (e.g., atrial fibrillation, atrial flutter, supraventricular tachycardia, atrial tachycardia). Monitor for bradycardia and hypotension when administering these medications.
Other miscellaneous drugs that may be used during cardiac arrest include sodium bicarbonate, calcium, and magnesium sulfate. Sodium bicarbonate is reserved for specific clinical situations including hyperkalemia, preexisting bicarbonate-responsive acidosis, and tricyclic antidepressant overdose. Magnesium is considered useful in the treatment of torsades de pointes, suspected hypomagnesaemia, and refractory ventricular fibrillation. Calcium, an ion essential for myocardial contractions and impulse formation, is recommended for hyperkalemia, hypocalcemia, and calcium channel blocker toxicity.
During cardiopulmonary arrest, hemodynamic status is unstable and requires intervention to stabilize not only the patient’s cardiac rhythm but also the cardiovascular system. Table 31-6 reviews vasoactive drugs more commonly used during cardiopulmonary emergencies. 1.9. and 22. Refer to Table 31-7 for an overview of cardiovascular drugs that can be given via the endotracheal tube or intraosseous route when IV access cannot be established. 1.9. and 22.
| ACE, Angiotensin-converting enzyme; AMI, acute myocardial infarction; BP, blood pressure; CHF, congestive heart failure; CV, cardiovascular; ECG, electrocardiogram; ET, endotracheal; HCL, hydrochloride; ICP, intracranial pressure; IVP, intravenous push; LR, lactated Ringer’s; PAT, paroxysmal atrial tachycardia; see Table 31-5 for other definitions. | |||||
| Drug | Category | Actions | Indications | Dose | Comments |
|---|---|---|---|---|---|
| Esmolol (Brevibloc) | β-Adrenergic blocker
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree

Get Clinical Tree app for offline access

| ||||