Section 3 Children’s nursing interventions
3.1 Interventions
Blood transfusion therapy
The Rhesus (Rh) system
The Rh D factor is significant in both blood transfusions and pregnancy:
Changes in blood begin within 24 hours of storage, and continue throughout the entire 21 days, after which blood is considered outdated (Edwards 1998). Box 3.1 show the changes that occur in stored blood.
Blood transfusion reactions (TF)
 When mismatched blood is infused, a transfusion reaction occurs and the donor and recipient’s red blood cells are attacked by the recipient’s immune system.
When mismatched blood is infused, a transfusion reaction occurs and the donor and recipient’s red blood cells are attacked by the recipient’s immune system.
 Transfusion reaction can occur with the infusion with as little as 10–15 ml.
Transfusion reaction can occur with the infusion with as little as 10–15 ml.
 The agglutination of the foreign red blood cells blocks small blood vessels throughout the body and is destroyed. This causes a reduction in the capacity of red blood cells to carry oxygen and obstruction to blood flow causing organ damage, both of which are lethal.
The agglutination of the foreign red blood cells blocks small blood vessels throughout the body and is destroyed. This causes a reduction in the capacity of red blood cells to carry oxygen and obstruction to blood flow causing organ damage, both of which are lethal.
If any serious adverse reaction or event occurs during or after a blood transfusion it should be reported on the serious adverse blood reactions and events (SABRE) system, accessed by the serious hazards of transfusion (SHOT) website at http://www.shotuk.org.
Nurses have to be vigilant in checking:
 The correct blood group and Rh D antigen factor
The correct blood group and Rh D antigen factor
 Confirming with the parent or child the identity
Confirming with the parent or child the identity
 Checking the ID band, e.g. date of birth, hospital number (no wristband – no transfusion)
Checking the ID band, e.g. date of birth, hospital number (no wristband – no transfusion)
 Donor number matches, CMV negative
Donor number matches, CMV negative
 Integrity of the bag, e.g. no damage has occurred
Integrity of the bag, e.g. no damage has occurred
 Kell or other antibodies/CMV negative
Kell or other antibodies/CMV negative
 Observing for any signs of transfusion reactions. If any of signs occur STOP the transfusion! Report the reaction
Observing for any signs of transfusion reactions. If any of signs occur STOP the transfusion! Report the reaction
Other blood product derivatives
 Packed red cells (whole blood from which most of the plasma has been removed) is generally only used to treat anaemia.
Packed red cells (whole blood from which most of the plasma has been removed) is generally only used to treat anaemia.
 Fresh frozen plasma (FFP) should never be used as a volume expander in this situation. It is better to use FFP for patients with bleeding disorders, whereby there is a deficiency in platelets or clotting factors, e.g. in disseminated intravascular coagulation (DIC), warfarin overdose, trauma or thrombotic thrombocytopenia.
Fresh frozen plasma (FFP) should never be used as a volume expander in this situation. It is better to use FFP for patients with bleeding disorders, whereby there is a deficiency in platelets or clotting factors, e.g. in disseminated intravascular coagulation (DIC), warfarin overdose, trauma or thrombotic thrombocytopenia.
For a more detailed account of blood products see Table 3.1.
Table 3.1 Current blood products
| Blood product | Constituents | Uses |
|---|---|---|
| Whole blood (510 ± 45 ml) | Use is restricted to circumstances where red blood cells as well as plasma proteins are needed i.e. where large amounts of blood are lost. | Ideal in hypovolaemic shock, since it increases both oxygen carrying capacity and expands circulating volume. |
| Packed cells (280 ± 60 ml) | This is whole blood, but the majority of the plasma has been removed. It contains half the volume of whole blood, less sodium, potassium, albumin and citrate. Does contain some white blood cells and platelets. | Ideal in chronic anaemia, sickle cell disease, thalassaemia and renal disease. It is not recommended in iron deficiency and vitamin B12 or folate deficiency as these should be treated with the appropriate vitamin e.g. iron tablets. |
| Washed packed cells | These are packed cells with all the white blood cells, platelets and plasma removed. | Indicated for patients who have a long history of transfusion reactions. |
| Fresh frozen plasma (FFP) (200–300 ml) | This is blood product, which is nearly always frozen and contains all the coagulation factors. | Used for the treatment of coagulation deficits. It is not recommended as a volume expander, except in certain neonatal conditions. |
| Cryoprecipitate (20 ± 5 ml) | Prepared from FFP and contains mainly clotting factors (factor VIII and fibrinogen). | Used to treat haemophilia or AIDS patients. |
| Platelets (50 ± 10 ml) | Produced from the residue left over from the production of plasma and leucocyte-depleted red blood cell concentrates. | Indications for use are thrombocytopenia, when platelet content of blood is reduced due to bleeding or diluted following massive transfusion, in acute leukaemia, aplastic anaemia, DIC or sepsis. |
AIDS, acquired immune deficiency syndrome; DIC, disseminated intravascular coagulation.
Synthetic blood products
 Artificial blood substitutes, or perfluorochemicals, have become available for clinical use.
Artificial blood substitutes, or perfluorochemicals, have become available for clinical use.
 Used in cases of severe anaemia when transfusion of blood products is not an option.
Used in cases of severe anaemia when transfusion of blood products is not an option.
 Use of these products to treat blood loss is still under investigation.
Use of these products to treat blood loss is still under investigation.
 When perfluorochemical microdroplets (which have a high solubility of oxygen) are infused intravenously, oxygen is dissolved in the microdroplets and transported to capillaries for diffusion across capillary walls (Edwards 1998).
When perfluorochemical microdroplets (which have a high solubility of oxygen) are infused intravenously, oxygen is dissolved in the microdroplets and transported to capillaries for diffusion across capillary walls (Edwards 1998).
Fluid replacement therapy
Crystalloid therapy
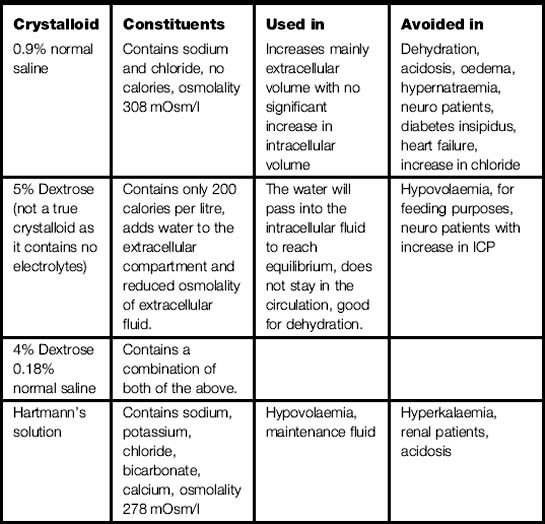
 Crystalloid solutions used are 5% dextrose (not a true crystalloid as it contains no electrolytes), 0.9% sodium chloride; dextrose saline and Hartmann’s (see Table 3.2).
Crystalloid solutions used are 5% dextrose (not a true crystalloid as it contains no electrolytes), 0.9% sodium chloride; dextrose saline and Hartmann’s (see Table 3.2).
 Any solution that contains electrolytes will influence fluid movement; the most powerful is sodium.
Any solution that contains electrolytes will influence fluid movement; the most powerful is sodium.
 Sodium added to the extracellular fluid compartment as in intravenous infusion will keep sodium at a normal level and have no effect on fluid movement but the infused solution of 0.9% normal saline/Hartmann’s solution will stay in the circulation and increase/maintain circulating volume.
Sodium added to the extracellular fluid compartment as in intravenous infusion will keep sodium at a normal level and have no effect on fluid movement but the infused solution of 0.9% normal saline/Hartmann’s solution will stay in the circulation and increase/maintain circulating volume.
Maintenance fluid
 It is extremely important to administer the correct volume.
It is extremely important to administer the correct volume.
 Devices should be used to administer the fluids and set at the correct rate over a specific period of time, and documented on a fluid balance chart.
Devices should be used to administer the fluids and set at the correct rate over a specific period of time, and documented on a fluid balance chart.
Some methods of calculating maintenance fluids:
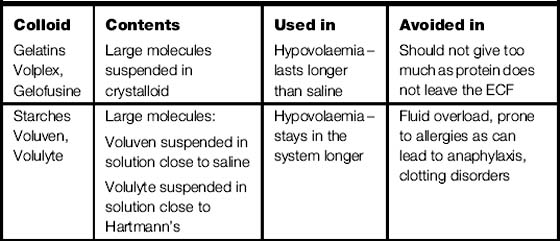
Colloid therapy
 Colloids work as they contain various amounts of large molecules, which draw fluid into the circulation from the intracellular spaces (the largest fluid compartment of the body), increasing circulating volume (volume expanders).
Colloids work as they contain various amounts of large molecules, which draw fluid into the circulation from the intracellular spaces (the largest fluid compartment of the body), increasing circulating volume (volume expanders).
 There are two different types of colloid gelatines and starches (see Table 3.3).
There are two different types of colloid gelatines and starches (see Table 3.3).
 Colloid supplementation, in the presence of normal kidney function, may aid the excretion of excess extracellular fluid.
Colloid supplementation, in the presence of normal kidney function, may aid the excretion of excess extracellular fluid.
 Using colloids in haemorrhage is to restore plasma volume, and improve or maintain oxygen transport.
Using colloids in haemorrhage is to restore plasma volume, and improve or maintain oxygen transport.
 Haemodynamic stability is achieved by increasing the blood volume by giving plasma expanders, and thus, providing adequate oxygen and nutrients, which are needed for the maintenance and restoration of cellular function.
Haemodynamic stability is achieved by increasing the blood volume by giving plasma expanders, and thus, providing adequate oxygen and nutrients, which are needed for the maintenance and restoration of cellular function.
 Inadequate circulatory blood volume, owing to haemorrhage, causes pooling of blood in the microcirculation, the major effect being a marked decrease in venous return, and a diminished cardiac output.
Inadequate circulatory blood volume, owing to haemorrhage, causes pooling of blood in the microcirculation, the major effect being a marked decrease in venous return, and a diminished cardiac output.
 By administering plasma expanders there is an improvement in oxygen availability, oxygen consumption, circulating volume, haemodynamic status and tissue perfusion.
By administering plasma expanders there is an improvement in oxygen availability, oxygen consumption, circulating volume, haemodynamic status and tissue perfusion.
 Colloid is not easily excreted by the kidneys as the renal tubules do not allow protein and other large molecules in the filtrate, thus the effect of can increase, giving rise to fluid overload.
Colloid is not easily excreted by the kidneys as the renal tubules do not allow protein and other large molecules in the filtrate, thus the effect of can increase, giving rise to fluid overload.
 Massive fluid replacement therapy of crystalloid or colloid can lead to the development of pulmonary oedema and haemodilution.
Massive fluid replacement therapy of crystalloid or colloid can lead to the development of pulmonary oedema and haemodilution.
Cannulation
A cannula is a vascular device inserted into a peripheral or central vessel to provide:
 relevant anatomy and physiology
relevant anatomy and physiology
 criteria for choosing vein and equipment
criteria for choosing vein and equipment
 health and safety regulations.
health and safety regulations.
 adherence to aseptic technique
adherence to aseptic technique
 adequate information regarding the procedure and complications.
adequate information regarding the procedure and complications.
Considerations when choosing a vein:
 injury, disease or treatment prevent use of a limb
injury, disease or treatment prevent use of a limb
 how the infant/child is positioned
how the infant/child is positioned
 if the infant/child is in shock or dehydrated poor superficial peripheral access may be present
if the infant/child is in shock or dehydrated poor superficial peripheral access may be present
 temperature will influence venous dilatation e.g. if the child is cold, no veins may be visible.
temperature will influence venous dilatation e.g. if the child is cold, no veins may be visible.
 medications can influence choice e.g. anticoagulants, steroids, risk of bruising.
medications can influence choice e.g. anticoagulants, steroids, risk of bruising.
 Consider whether cannula is actually necessary.
Consider whether cannula is actually necessary.
 Child will be anxious about the procedure and pain. Thus it is essential to use local anaesthetic prior to procedure.
Child will be anxious about the procedure and pain. Thus it is essential to use local anaesthetic prior to procedure.
 Choose a site away from a joint.
Choose a site away from a joint.
 Prevent infection of skin flora contamination from nurse to child:
Prevent infection of skin flora contamination from nurse to child:
 Reduce the number of attempts to cannulate as increased number of puncture sites equates increased entry sites for infection.
Reduce the number of attempts to cannulate as increased number of puncture sites equates increased entry sites for infection.
 Check patency using normal saline.
Check patency using normal saline.
 Cover the cannula with a dressing, which is, transparent and semipermeable, allows the site to be viewed easily.
Cover the cannula with a dressing, which is, transparent and semipermeable, allows the site to be viewed easily.
 Change the cannula site every 48–72 hours – this has been shown to reduce infection rates at the cannula site.
Change the cannula site every 48–72 hours – this has been shown to reduce infection rates at the cannula site.
Insertion site checked regularly for signs of:
Table 3.4 Infiltration scoring system
| Grade | Clinical criteria |
|---|---|
| 0 | No symptoms |
| 1 | Skin blanched Oedema 2.5 cm in any direction Cool to touch With or without pain |
| 2 | Skin blanched Oedema 2.5–15 cm in any direction Cool to touch With or without pain |
| 3 | Skin blanched, translucent Gross oedema 15 cm in any direction Cool to touch Mild to moderate pain Possible numbness |
| 4 | Skin blanched, translucent Skin tight, leaking Skin discoloured, bruised, swollen Gross oedema 15 cm in any direction Deep pitting tissue oedema Circulatory impairment Moderate to severe pain Infiltration of any amount of blood product, irritant or vesicant |
Phlebitis
 Acute inflammation of a vein directly linked to the presence of any vascular device.
Acute inflammation of a vein directly linked to the presence of any vascular device.
 Infants may cry or children may report pain.
Infants may cry or children may report pain.
 If treated early enough often the symptoms will resolve without further intervention required.
If treated early enough often the symptoms will resolve without further intervention required.
 Substances that often cause phlebitis tend to be isotonic solutions, e.g. 0.9% sodium chloride (NaCl) or blood products.
Substances that often cause phlebitis tend to be isotonic solutions, e.g. 0.9% sodium chloride (NaCl) or blood products.
 Phlebitis can be further classified into mechanical, chemical and infective, depending on the cause:
Phlebitis can be further classified into mechanical, chemical and infective, depending on the cause:
 Treatment is removal of the line and the application of heat to the site and prescribed analgesia
Treatment is removal of the line and the application of heat to the site and prescribed analgesia
Flushing of cannula
 Flushing guidance is often overlooked, and is an essential component of good care.
Flushing guidance is often overlooked, and is an essential component of good care.
 Flush volume should be equal to at least twice the volume of the catheter, usually 5–10 ml of 0.9% NaCl (RCN 2003)
Flush volume should be equal to at least twice the volume of the catheter, usually 5–10 ml of 0.9% NaCl (RCN 2003)
 An associated hazard is speed shock as a systemic reaction that occurs when a substance foreign to the body is rapidly introduced – occurs most commonly with rapid bolus injection.
An associated hazard is speed shock as a systemic reaction that occurs when a substance foreign to the body is rapidly introduced – occurs most commonly with rapid bolus injection.
Anaphylaxis
 With increased use of medicines and antibacterials, medicine induced anaphylaxis and anaphylactoid reactions have increased.
With increased use of medicines and antibacterials, medicine induced anaphylaxis and anaphylactoid reactions have increased.
 Other causative agents include; NSAIDs, anaesthetics, muscle relaxants, latex and radio contrast media.
Other causative agents include; NSAIDs, anaesthetics, muscle relaxants, latex and radio contrast media.
Anaphylaxis is often unpredictable and so we need to focus on strategies to decrease risks:
 Ensure detailed patient history and full physical examination.
Ensure detailed patient history and full physical examination.
 Consider the route of the medicine and the rate of the medicine and/or fluid.
Consider the route of the medicine and the rate of the medicine and/or fluid.
 Identification of patients with known causes of anaphylaxis.
Identification of patients with known causes of anaphylaxis.
 Sound knowledge of the medicine, as some cross react and also are contraindicated.
Sound knowledge of the medicine, as some cross react and also are contraindicated.
 Greater number of years since the last administration of the offending agent, the less the chance of a recurrence.
Greater number of years since the last administration of the offending agent, the less the chance of a recurrence.
 Parenteral route increases the severity and frequency of a reaction, so review the choice of the medicine and route, and if the patient still needs IV route, they remain under medical supervision for 20–30 minutes after medicine administration.
Parenteral route increases the severity and frequency of a reaction, so review the choice of the medicine and route, and if the patient still needs IV route, they remain under medical supervision for 20–30 minutes after medicine administration.
Recommendations for practice
 Discontinue suspected medicine.
Discontinue suspected medicine.
 Administer oxygen, epinephrine (adrenaline), IV fluids.
Administer oxygen, epinephrine (adrenaline), IV fluids.
 Monitor patient; oxygen saturation, vital signs, ECG.
Monitor patient; oxygen saturation, vital signs, ECG.
 Provide reassurance and adequate information and communication.
Provide reassurance and adequate information and communication.
 At least 2 hours observation if mild and in severe cases at least 24 hours monitoring of the patient.
At least 2 hours observation if mild and in severe cases at least 24 hours monitoring of the patient.
 Ensure prompt and appropriate reporting and recording in the patient case records and consideration of the Yellow Card Reporting scheme, which advocates the reporting of adverse medicine reactions.
Ensure prompt and appropriate reporting and recording in the patient case records and consideration of the Yellow Card Reporting scheme, which advocates the reporting of adverse medicine reactions.
 Patient will require advice for the future and this might include: the use of a Medic-Alert (e.g. bracelet) ID system and an Epinephrine kit.
Patient will require advice for the future and this might include: the use of a Medic-Alert (e.g. bracelet) ID system and an Epinephrine kit.
Oxygen therapy
Hypoxia is oxygen deficiency in the body cells caused by:
 Deficient oxygenation of the blood – due to respiratory disease or chest injuries.
Deficient oxygenation of the blood – due to respiratory disease or chest injuries.
 Inadequate transport of oxygen by haemoglobin – as in anaemia or haemorrhage.
Inadequate transport of oxygen by haemoglobin – as in anaemia or haemorrhage.
 Circulatory inadequacy as in heart disease or emergency situations, e.g. cardiac arrest.
Circulatory inadequacy as in heart disease or emergency situations, e.g. cardiac arrest.
 Inability of cells to use oxygen – rare – an example is cyanide poisoning.
Inability of cells to use oxygen – rare – an example is cyanide poisoning.
 Oxygen toxicity – may follow prolonged periods (over 24 hours) of administration of high (over 50%) concentrations of oxygen.
Oxygen toxicity – may follow prolonged periods (over 24 hours) of administration of high (over 50%) concentrations of oxygen.
 Atelectasis – alveolar collapse increases with oxygen greater than 50%, as nitrogen is washed out and replaced by oxygen.
Atelectasis – alveolar collapse increases with oxygen greater than 50%, as nitrogen is washed out and replaced by oxygen.
 Retinopathy or prematurity – high concentrations of oxygen obliterates developing retinal vessels leading to blindness.
Retinopathy or prematurity – high concentrations of oxygen obliterates developing retinal vessels leading to blindness.
Humidified oxygen
Indications for the use of heated humidification systems:
 Patients receiving high concentrations of oxygen (where FiO2 exceeds 40%) for periods of time exceeding 24 hours.
Patients receiving high concentrations of oxygen (where FiO2 exceeds 40%) for periods of time exceeding 24 hours.
 Patients with conditions causing poor mucociliary transport, sometimes found in patients with severe inflammation of the oropharyngeal mucosa.
Patients with conditions causing poor mucociliary transport, sometimes found in patients with severe inflammation of the oropharyngeal mucosa.
 Patients with hypothermia. Heated humidified oxygen therapy may help increase a patient’s core body temperature when used in combination with other treatments.
Patients with hypothermia. Heated humidified oxygen therapy may help increase a patient’s core body temperature when used in combination with other treatments.
 Humidified oxygen therapy is the process of delivering moisture inspired oxygen to patients receiving mechanical ventilation, non-invasive ventilatory support or who are able to breathe independently but require humidified oxygen.
Humidified oxygen therapy is the process of delivering moisture inspired oxygen to patients receiving mechanical ventilation, non-invasive ventilatory support or who are able to breathe independently but require humidified oxygen.
 Humidified oxygen can be delivered as a heat and moisture exchanger (HME) or in the form of heated humidification.
Humidified oxygen can be delivered as a heat and moisture exchanger (HME) or in the form of heated humidification.
Heated humidification
 secretions (chronic bronchitis, cystic fibrosis, etc.)
secretions (chronic bronchitis, cystic fibrosis, etc.)
 bloody secretions (pulmonary contusion or haemorrhage), very high or very low tidal volumes
bloody secretions (pulmonary contusion or haemorrhage), very high or very low tidal volumes
 spontaneous minute ventilation which exceeds 10 l/min, during hypothermia (body temperature less than 32°C),
spontaneous minute ventilation which exceeds 10 l/min, during hypothermia (body temperature less than 32°C),
 patients requiring ventilation exceeding 96 hours.
patients requiring ventilation exceeding 96 hours.
Rewarming procedures
Passive rewarming
 Once the patient’s core temperature is less than 35°C, steps should be taken to prevent them losing further heat to the environment. Remove all wet clothing; gently dry the patient, if needed then insulate them with blankets. Patient is allowed to rewarm using just normal metabolic heat production.
Once the patient’s core temperature is less than 35°C, steps should be taken to prevent them losing further heat to the environment. Remove all wet clothing; gently dry the patient, if needed then insulate them with blankets. Patient is allowed to rewarm using just normal metabolic heat production.
 Patient may be covered with polythene sheeting, and placed in a warm room. Space blankets prevent radiant heat loss, but not that lost by conduction or convection. As these may cause sparks, they also present a hazard when oxygen is used, so are best avoided. Remember that heat is lost from the head and the back, so insulate these areas.
Patient may be covered with polythene sheeting, and placed in a warm room. Space blankets prevent radiant heat loss, but not that lost by conduction or convection. As these may cause sparks, they also present a hazard when oxygen is used, so are best avoided. Remember that heat is lost from the head and the back, so insulate these areas.
 This method is recommended for both mild (32.2–35°C) and moderate (28–32°C) hypothermias that have an onset of less than 12 hours. Passive rewarming treatment will not rewarm an arrested hypothermic patient, and may have limited value for those who are severely hypothermic e.g. a temperature of less than 28°C.
This method is recommended for both mild (32.2–35°C) and moderate (28–32°C) hypothermias that have an onset of less than 12 hours. Passive rewarming treatment will not rewarm an arrested hypothermic patient, and may have limited value for those who are severely hypothermic e.g. a temperature of less than 28°C.
 Close observation, cautious use of fluids and avoidance of vigorous movement to prevent cardiac arrest are essential. Movement contributes to heat loss through convection and may reduce temperature further if not closely monitored. If the temperature fails to rise, and patient become persistently hypotensive, active external rewarming should be commenced.
Close observation, cautious use of fluids and avoidance of vigorous movement to prevent cardiac arrest are essential. Movement contributes to heat loss through convection and may reduce temperature further if not closely monitored. If the temperature fails to rise, and patient become persistently hypotensive, active external rewarming should be commenced.
Active external rewarming
 Patient’s skin is warmed, using hot baths, hot air blowers or radiant heat. This method can also be used as an adjunct to internal active rewarming. One of the most effective methods is convective warming therapy which forces heated air directly on to the patient’s skin through a disposable blanket.
Patient’s skin is warmed, using hot baths, hot air blowers or radiant heat. This method can also be used as an adjunct to internal active rewarming. One of the most effective methods is convective warming therapy which forces heated air directly on to the patient’s skin through a disposable blanket.
 Method may be used when the hypothermia has occurred slowly, e.g. over a 12-hour period and is mild or moderate in nature. It is not recommended for use alone in the treatment of patients with severe hypothermia.
Method may be used when the hypothermia has occurred slowly, e.g. over a 12-hour period and is mild or moderate in nature. It is not recommended for use alone in the treatment of patients with severe hypothermia.
 Patient’s vital signs and peripheral temperature must be monitored, as rewarming shock may occur in the severely hypothermic patient as a consequence of rewarming the peripheries before the core.
Patient’s vital signs and peripheral temperature must be monitored, as rewarming shock may occur in the severely hypothermic patient as a consequence of rewarming the peripheries before the core.
 Peripheral rewarming promotes vasodilatation, returning cold, acidotic blood to the heart that has significant effects on myocardial depression. To minimize this effect it is recommended that only truncal rewarming be undertaken. If the patient is persistently hypotensive, or their core temperature continues to fall, active internal rewarming should be started.
Peripheral rewarming promotes vasodilatation, returning cold, acidotic blood to the heart that has significant effects on myocardial depression. To minimize this effect it is recommended that only truncal rewarming be undertaken. If the patient is persistently hypotensive, or their core temperature continues to fall, active internal rewarming should be started.
Active internal rewarming
 warm fluid for gastric and peritoneal lavage
warm fluid for gastric and peritoneal lavage
 mediastinal and pleural irrigation
mediastinal and pleural irrigation
 continuous arteriovenous or venovenous rewarming
continuous arteriovenous or venovenous rewarming
 The advantage of active core rewarming is that it avoids the peripheral vasodilatation associated with surface rewarming, and allows correction of any fluid deficits.
The advantage of active core rewarming is that it avoids the peripheral vasodilatation associated with surface rewarming, and allows correction of any fluid deficits.
 The disadvantage is that afterdrop may be observed in patients after internal active rewarming is discontinued. A decrease in temperature of as much as 2°C may occur as blood circulates to the peripheries, recools, and returns to the core. Thus, when more invasive active internal rewarming methods are discontinued, attention is directed to the need for passive and active external rewarming, to prevent afterdrop in temperature.
The disadvantage is that afterdrop may be observed in patients after internal active rewarming is discontinued. A decrease in temperature of as much as 2°C may occur as blood circulates to the peripheries, recools, and returns to the core. Thus, when more invasive active internal rewarming methods are discontinued, attention is directed to the need for passive and active external rewarming, to prevent afterdrop in temperature.
 the method is best incorporated when the hypothermia has occurred very quickly, e.g. in less than 12 hours, and is moderate or severe in nature. The treatment is to reduce the risk of cardiac arrest, by reducing the time the patient’s core temperature is below 32·2°C.
the method is best incorporated when the hypothermia has occurred very quickly, e.g. in less than 12 hours, and is moderate or severe in nature. The treatment is to reduce the risk of cardiac arrest, by reducing the time the patient’s core temperature is below 32·2°C.
 Process of rewarming should proceed at no faster than a few degrees per hour (Edwards 2003). If a patient is rapidly rewarmed oxygen consumption, myocardial demand and vasodilatation increase faster than the heart’s ability to compensate and death can occur.
Process of rewarming should proceed at no faster than a few degrees per hour (Edwards 2003). If a patient is rapidly rewarmed oxygen consumption, myocardial demand and vasodilatation increase faster than the heart’s ability to compensate and death can occur.
3.2 Maintaining nutrition in children
Effects of nil by mouth and malnutrition
Absorptive and post-absorptive states:
 Absorptive state – process of eating/digestion
Absorptive state – process of eating/digestion
 Post-absorptive state – fasting should be no more than 12 hours as after this time:
Post-absorptive state – fasting should be no more than 12 hours as after this time:
 Reduction in the production in white blood cells, immunoglobulins – susceptibility to infection.
Reduction in the production in white blood cells, immunoglobulins – susceptibility to infection.
 Reduction in adeno-triphosphate (ATP) in skeletal muscle cells leading to weakness, lethargy, reduced mobility – potential deep vein thrombosis and pulmonary embolism.
Reduction in adeno-triphosphate (ATP) in skeletal muscle cells leading to weakness, lethargy, reduced mobility – potential deep vein thrombosis and pulmonary embolism.
 Reduction in ATP can also lead to weakened diaphragm and muscles of breathing which can lead to a chest infection or pneumonia.
Reduction in ATP can also lead to weakened diaphragm and muscles of breathing which can lead to a chest infection or pneumonia.
Enteral feeding (EF)
 reduces risk of bacterial translocation
reduces risk of bacterial translocation
 dampens the inflammatory response
dampens the inflammatory response
 reduces complications from sepsis
reduces complications from sepsis
Types of tubes
 Wide bore tube is used initially to allow easy assessment of gastric contents and aspiration typically occurs every 4 hours to assess gastric content/absorption and pH.
Wide bore tube is used initially to allow easy assessment of gastric contents and aspiration typically occurs every 4 hours to assess gastric content/absorption and pH.
 Narrow bore tube should replace the wide bore tube to facilitate long-tem feeding. In addition, the narrow bore tube is more comfortable for the patient and less likely to cause oesophageal irritation or interfere with swallowing.
Narrow bore tube should replace the wide bore tube to facilitate long-tem feeding. In addition, the narrow bore tube is more comfortable for the patient and less likely to cause oesophageal irritation or interfere with swallowing.
 Percutaneous endoscopically gastrostomy (PEG) –made from polyurethane or silicone and held in place by an inflatable balloon. Disadvantage is that it requires local anaesthetic, sedation and radiological support to ensure tube is positioned correctly.
Percutaneous endoscopically gastrostomy (PEG) –made from polyurethane or silicone and held in place by an inflatable balloon. Disadvantage is that it requires local anaesthetic, sedation and radiological support to ensure tube is positioned correctly.
 Jejunostomy tube – placed in the jejunum and is the preferable method if the patient has undergone upper gastrointestinal surgery or has severe delayed gastric emptying.
Jejunostomy tube – placed in the jejunum and is the preferable method if the patient has undergone upper gastrointestinal surgery or has severe delayed gastric emptying.
Methods of administration
 Bolus feeding: if the patient is restless or confused as the patient may dislodge the tube.
Bolus feeding: if the patient is restless or confused as the patient may dislodge the tube.
 Intermittent continuous feeding: if feeding needs to be interrupted or discontinued to allow gastric emptying, for example for physiotherapy.
Intermittent continuous feeding: if feeding needs to be interrupted or discontinued to allow gastric emptying, for example for physiotherapy.
 Gravity drip –allowed to flow through over a given period of time
Gravity drip –allowed to flow through over a given period of time
 Pump assisted feeding – connected to a pump for the majority of the day and typically rested overnight. Most commonly used in the critical care setting, various flow rates per hour from 1 ml to 300 ml.
Pump assisted feeding – connected to a pump for the majority of the day and typically rested overnight. Most commonly used in the critical care setting, various flow rates per hour from 1 ml to 300 ml.
Complications of enteral feeding (EF)
 Altered gut motility, that is absent bowel sounds can lead to gastric and colonic stasis.
Altered gut motility, that is absent bowel sounds can lead to gastric and colonic stasis.
 Effects of sedation and analgesia can lead to ileus/pseudo obstruction and distension.
Effects of sedation and analgesia can lead to ileus/pseudo obstruction and distension.
 Diarrhoea, constipation, large aspirates.
Diarrhoea, constipation, large aspirates.
 Incomplete calorific delivery due to the above.
Incomplete calorific delivery due to the above.
Strategies to avoid complications associated with EF:
 Large aspirates – give prokinetic agents, e.g. pantoprazole.
Large aspirates – give prokinetic agents, e.g. pantoprazole.
 Early commencement of EF – day 1 of critical care admission, monitor feeding regime, avoid stopping and starting feed, follow feeding regime.
Early commencement of EF – day 1 of critical care admission, monitor feeding regime, avoid stopping and starting feed, follow feeding regime.
 Care of nasogastric tube to prevent blockages.
Care of nasogastric tube to prevent blockages.
 Bowel sounds are not required for enteral feeding to commence.
Bowel sounds are not required for enteral feeding to commence.
 Treat diarrhoea, constipation and vomiting to aid enteral feeding.
Treat diarrhoea, constipation and vomiting to aid enteral feeding.