Section Twenty-Four Medication Administration
PROCEDURE 176 Nitrous Oxide Administration
PROCEDURE 178 Peripheral Nerve Stimulator
PROCEDURE 179 Streptokinase Administration
PROCEDURE 180 TNK-tPA Administration for Acute Myocardial Infarction
PROCEDURE 181 tPA Administration for Acute Myocardial Infarction or Pulmonary Embolus
PROCEDURE 182 tPA Administration for Acute Ischemic Stroke
PROCEDURE 183 Reteplase Administration for Acute Myocardial Infarction
PROCEDURE 184 High-dose Steroids for Spinal Cord Injury
PROCEDURE 185 Administration of Methotrexate for Ectopic Pregnancy
PROCEDURE 176 Nitrous Oxide Administration
Nitrous oxide combined with oxygen is also known as laughing gas, Nitronox, and Entonox.
INDICATIONS
1. To provide rapid-onset (2 to 6 minutes), quickly reversible (2 to 5 minutes) analgesia for 30 minutes or less. Nitrous oxide may be used to relieve pain associated with trauma, renal colic, myocardial infarction, minor surgical procedures, wound care, diagnostic procedures, and reduction of fractures and dislocations.
2. To relieve the anxiety associated with painful conditions and procedures
CONTRAINDICATIONS AND CAUTIONS
1. When used for analgesia in the emergency setting (versus anesthesia in the operative setting), the gas mixture should be fixed at 50% nitrous oxide to 50% oxygen. For altitudes above 3500 feet, a 65% nitrous oxide to 35% oxygen mixture is recommended because the lower partial pressure of the nitrous oxide at higher altitudes does not provide adequate analgesia.
2. The patient should always self-administer the gas to prevent oversedation. Therefore, the patient must be cooperative and able to follow instructions. The mask or mouthpiece should never be strapped to or held on the patient’s face.
3. Nitrous oxide causes drowsiness and should not be used in patients with altered levels of consciousness or head injuries, or those who are heavily sedated or intoxicated. Patients who have received opioids should be individually evaluated for suitability before receiving nitrous oxide.
4. Nitrous oxide occasionally causes nausea and vomiting, so caution is indicated with recent ingestion of food or fluids.
5. The gas mixture contains 50% oxygen (35% at high altitude); therefore, it does not supply enough oxygen for patients who have pulmonary edema and may suppress the hypoxic respiratory drive in a patient who has chronic obstructive pulmonary disease.
6. Nitrous oxide collects in dead air spaces and can expand the preexisting pockets of air associated with pneumothorax, otitis media, perforated viscus, bowel obstruction, air embolism, and decompression sickness.
7. Nitrous oxide should not be used during early pregnancy, because it has been associated with fetal defects and spontaneous abortion.
8. A scavenger system to dispose of exhaled gas protects health care providers who administer nitrous oxide. Studies involving operating room and dental personnel have associated long-term exposure to nitrous oxide with psychomotor impairment and congenital malformations, as well as spontaneous abortion in exposed women and sexual partners of exposed men. Possible association with bone marrow suppression, cancer, liver disease, and renal disease has also been reported.
9. A mask is preferred to facilitate disposal of exhaled gas. If the patient is unable to use a mask because of facial trauma or other conditions, a mouthpiece may be used in place of a mask.
10. Nitrous oxide has abuse potential. The unit should be kept in a secure area. A pop-off demand valve is available; the valve can then be locked up with the narcotics.
11. A fail-safe valve should be incorporated into the system to prevent administration of 100% nitrous oxide if the oxygen supply is interrupted.
12. A nurse or physician should be present at all times during nitrous oxide administration in the hospital setting unless institutional policies are in place specifying other qualified personnel who may monitor the patient.
EQUIPMENT
Nitrous oxide and oxygen tanks connected to a blender preset to deliver 50% nitrous oxide and 50% oxygen (65% nitrous oxide and 35% oxygen at altitudes above 3500 feet) with demand valve and scavenger (Figure 176-1). (Units are also available for use in the prehospital environment and for nitrous oxide supplied by pipeline.)
Wall suction and suction connection tubing
NOTE: In countries other than the United States, nitrous oxide may be available in a single-tank mixture known as Entonox.
PATIENT PREPARATION
PROCEDURAL STEPS
1. Turn on the nitrous oxide and oxygen tanks by opening the cylinder valves with a wrench. Check that the mixture pressure is within the safe level per manufacturer’s specifications (30 to 35 psi for Nitronox by Matrx Medical, Inc. [1996]). Do not use the unit if the mixture pressure is not within the safe level. Check the pressure of each cylinder, and replace any cylinder with a pressure less than 300 psi (see Procedure 26).
2. Connect the scavenger to the wall suction and turn the suction on. Turn the ball valve lever on the scavenger tube to the ON position. Adjust the suction to 30 to 60 L/min.
3. Attach mask or mouthpiece to the demand valve.
4. Allow the patient to inhale the gas for 3 or 4 minutes before beginning any procedures. Some patients may require up to 6 minutes for adequate induction.
5. Monitor pulse oximetry continuously, and document it frequently (i.e., every 5 minutes) throughout the procedure.
6. As the patient becomes relaxed, he or she will be unable to create an adequate amount of negative pressure to trip the demand valve. This prevents excessive sedation.
7. When the patient drops the mask or mouthpiece, position or hold it so that the exhaled gas can be taken up by the scavenger.
8. Allow the patient to resume gas inhalation when he or she is able to hold the mask or mouthpiece.
9. Nitrous oxide administration is usually limited to a maximum of 30 minutes.
10. Assess and document vital signs and pulse oximetry.
11. When the procedure is completed, turn off the suction and nitrous and oxygen tanks. Clear the lines by blowing forcefully through the small holes on the back of the demand valve until all pressure gauges read zero. If the oxygen line bleeds before the nitrous line, you will not be able to remove the nitrous. Open and close the oxygen line and try again.
12. Monitor pulse oximetry and heart rate for 5 to 15 minutes after procedure.
NOTE: Some sources recommend that the patient receive supplemental oxygen for a period of time after receiving nitrous oxide to counteract the effects of diffusion hypoxia caused by the diffusion of nitrous oxide from the arterial blood into the alveoli, which dilutes alveolar oxygen levels. Research has demonstrated that diffusion hypoxia does not occur in healthy subjects after self-administration of nitrous oxide analgesia (Holcomb, Erdmann, & Corssen, 1976; Nieto & Rosen, 1980; Stewart, Gorayeb, & Pelton, 1986).
COMPLICATIONS
1. Side effects are unusual but may require termination of nitrous oxide administration. Side effects may include vomiting, shortness of breath, excitement, drowsiness, confusion, and light-headedness.
2. Propping or holding the mask against the patient’s face may result in excessive sedation. The patient must hold the mask to prevent overdosage.
3. Aspiration may occur if the patient vomits with the mask in place.
4. The Nitronox unit has a mixer pressure whistle alarm that sounds if there is a disruption in gas pressures resulting in a low oxygen concentration. Discontinue use immediately and notify the manufacturer (Matrx, 1996).
Holcomb C., Erdmann W., Corssen G. The significance of diffusion hypoxemia. Southern Medical Journal. 1976;69:1282–1284.
Matrx Medical, Inc. Instructions Nitronox hospital model. Orchard Park, NY: Author, 1996.
Nieto J., Rosen P. Nitrous oxide at higher elevations. Annals of Emergency Medicine. 1980;9:610–612.
Stewart R.D., Gorayeb M.J., Pelton G.H. Arterial blood gases before, during, and after nitrous oxide administration. Annals of Emergency Medicine. 1986;15:1177–1180.
PROCEDURE 177 Sedation
Sedation is also known as conscious sedation and moderate sedation. The current term for sedation is procedural sedation and analgesia (PSA). PSA encompasses a continuum that ranges from minimal sedation to general anesthesia, with distinct criteria for each level (Bahn, 2005).
INDICATIONS
To allay patient fear, anxiety, and pain and to improve the patient’s ability to cooperate during painful therapeutic, diagnostic, or surgical procedures. Procedural sedation is the administration of sedatives or dissociative agents (with or without analgesics) which will allow the patient to tolerate painful or unpleasant procedures while maintaining normal cardiorespiratory function and independent airway control (ACEP, 2005; Bahn, 2005).
CONTRAINDICATIONS AND CAUTIONS
1. Intoxicated with central nervous system depressants
3. Concurrent shock or myocardial infarction
4. Adrenal insufficiency or long-term steroid use (premedicate with intravenous steroid)
5. Moderate-to-severe liver or renal insufficiency
7. Monoamine oxidase inhibitor use within the previous 2 weeks
8. Any condition that could make intubation difficult (e.g., facial or neck trauma, large tongue, short neck, congenital malformation of upper airway structures)
9. ASA Patient Physical Status Classification greater than Class 2 (Table 177-1)
Table 177-1 AMERICAN SOCIETY OF ANESTHESIOLOGISTS’ PHYSICAL STATUS CLASSIFICATION SYSTEM
| Class | Description | Suitability for Sedation |
|---|---|---|
| P1 | Normal healthy patient | Excellent |
| P2 | Mild systemic disease; well-controlled chronic condition | Generally good |
| P3 | Severe systemic disease; poorly controlled chronic condition; acute illness such as pneumonia | Intermediate |
| P4 | Severe systemic disease that is a constant threat to life | Poor |
| P5 | Moribund patient who is not expected to survive without the operation | Extremely poor |
From American Society of Anesthesiologists. (n.d.) ASA physical status classification system. Retrieved February 17, 2007, from http://www.asahq.org/clinical/physicalstatus.htm; and Doyle, L. (2006). Pediatric procedural sedation and analgesia. Pediatric Clinics of North America, 53, 279–292.
EQUIPMENT
Oral and nasopharyngeal airways (appropriate size for patient)
Supplemental oxygen source, oxygen cannula, and mask (appropriate size for patient)
Bag-mask (appropriate size for patient)
Cardiac monitor (if indicated by patient’s condition)
Blood pressure monitoring equipment
Reversal agent(s) for medications to be administered (naloxone, nalmefene, and/or flumazenil)
PATIENT PREPARATION
1. Establish intravenous access (see Procedure 60). Intravenous access must be continuously maintained to provide a method of administering sedatives and initiating corrective action for adverse effects. For other routes of medication administration, you must have the ability to obtain intravenous access immediately (ASA, 2002).
2. Initiate pulse oximetry monitoring (see Procedure 21). Initiate end tidal CO2, cardiac, and blood pressure monitoring as indicated (see Procedures 24 and 55).
PROCEDURAL STEPS
1. * Before the administration of sedatives, complete a preanesthesia assessment, with documentation, to include at least the following (ASA, 2002):
2. Assess baseline physiologic status of patient including, but not limited to, the following:
3. Have the following available in the procedure area before the administration of any medications: an emergency cart with defibrillator, suction, airway management devices, and reversal agents for the medications being administered.
4. Have supplemental oxygen and suction immediately available.
5. Establish provisions for backup personnel who are experts in airway management and cardiopulmonary resuscitation in the event that complications arise.
6. Maintain a sedation checklist to complete all requirements and record frequently monitored physiologic parameters. Documentation should include the following (AORN, 2002):
7. Monitor the patient carefully from the administration of the medications until recovery. The nurse monitoring the patient should have no other responsibilities during or after the procedure that will interfere the nurse’s ability to adequately monitor the patient (AORN, 2002; ASA, 2002; ENA & ACEP, 2005).
8. During the procedure, document the following physiologic parameters at regular intervals (usually every 5 to 15 minutes, consult your institutional policy) (ASA, 2002):
9. After the procedure, document the patient’s level of consciousness, vital signs, and pulse oximetry every 15 minutes until he or she has recovered. Recovery is indicated by stable vital signs and oxygen saturation and alert and oriented state (or return to baseline mental status). Before discharge, the patient should be able to sit and ambulate (age appropriate) and tolerate oral fluids (ASA, 2002).
SAMPLE QUALIFICATIONS FOR ADMINISTERING AND MONITORING SEDATION
The nurse monitoring the patient must be competent in the use of resuscitation and monitoring equipment and should be able to interpret the data obtained (AORN, 2002; ENA, ACEP, 2005). This includes the ability to identify, rescue, and support a patient who slips into deep sedation and who may not be able to maintain a patent airway and adequate ventilation without assistance. Education in and demonstration of these competencies may be accomplished via the following:
1. Advanced cardiac life support (ACLS)
3. Training in the recognition of the cardiovascular and respiratory side effects of sedatives as well as the variability of patient response
4. Training in airway management
5. Emergency nursing pediatric course (ENPC) or pediatric advanced life support (PALS) for sedation of children
6. Knowledge of the pharmacology of the medications administered
AGE-SPECIFIC CONSIDERATIONS
1. Consider dosage reduction in the elderly and chronically ill.
2. When selecting the medications for a pediatric patient, consider the nature of the procedure (painful or nonpainful), the desired onset and duration of action, and the route of administration. In children, moderate sedation may not be adequate, and deep sedation may be the goal. Careful monitoring is indicated.
COMPLICATIONS
PATIENT TEACHING
1. Stay with a responsible adult for 12 hours after the procedure.
2. Do not drive an automobile or operate dangerous machinery for at least 6 to 12 hours. Children should not climb stairs unassisted, ride bikes, or play on playground equipment.
3. Return to the emergency department or call an ambulance for difficulty in breathing, pale or gray skin, or difficulty arousing.
4. Call the physician if vomiting is persistent and fluids do not stay down.
American College of Emergency Physicians (ACEP). Clinical policy for procedural sedation and analgesia in the emergency department. Annals of Emergency Medicine. 2005;31:663–677.
American Society of Anesthesiologists (ASA). Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017.
American Society of Anesthesiologists (ASA). (n.d.) ASA physical status classification system. Retrieved February 17, 2007, from http://www.asahq.org/clinical/physicalstatus.htm
Association of Operating Room Nurses (AORN). Recommended practices for managing the patient receiving moderate sedation. AORN Journal. 2002;75:642–652.
Bahn E.L. Procedural sedation and analgesia: A review and new concepts. Emergency Medicine Clinics of North America. 2005;23:503–517.
Doyle L. Pediatric procedural sedation and analgesia. Pediatric Clinics of North America. 2006;53:279–292.
Emergency Nurses Association (ENA) & American College of Emergency Physicians (ACEP). (2005). Delivery of agents for procedural sedation and analgesia by emergency nurses: Joint ENA/ACEP Statement. Retrieved January 29, 2007, from http://www.ena.org/about/position/ACEP/ProceduralSedation.asp
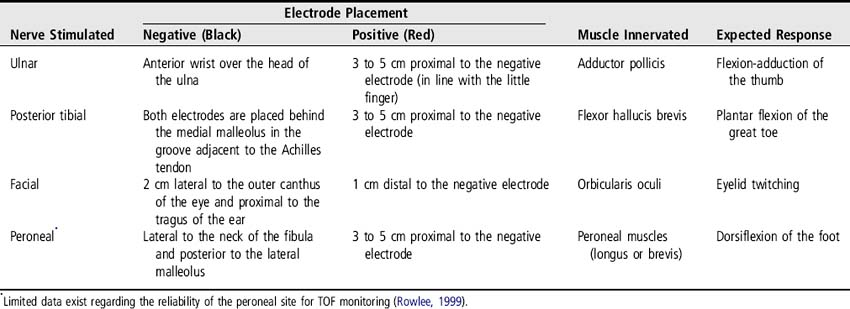
PROCEDURE 178 Peripheral Nerve Stimulator
Peripheral nerve stimulator is also known as twitch monitor or train of four (TOF) stimulator.
CONTRAINDICATIONS AND CAUTIONS
1. There are no absolute contraindications for TOF monitoring; however, patients with physiologic paralysis or degenerative neuromuscular disorders may not have normal muscle contractions in response to peripheral nerve stimulation.
2. Diaphoresis may impede electrode contact altering the muscle response to stimulation.
3. Low batteries may result in less than desired stimulus magnitude.
4. Significant edema or previous nerve damage may produce an altered muscle response.
PATIENT PREPARATION
Select the site for electrode placement; the ulnar nerve is the preferred site. The posterior tibial nerve is an acceptable site if the ulnar nerve is not an option.
PROCEDURAL STEPS
1. Apply electrodes at the selected site (Figure 178-1 and Table 178-1).
2. Attach the peripheral nerve stimulator leads to electrodes. The negative (black) electrode should be placed most distal when using the ulnar nerve site (UNC, 2005).
3. Turn the peripheral nerve stimulator on and set the mA at 10.
4. Push the TOF button and observe for the expected movement (see Table 178-1). Obtain the baseline TOF level by increasing the threshold (amplitude) by increments of 10 mA (to a maximum of 30) until four distinct muscle contractions are observed. If the expected muscle contractions are not seen at 30 mA, check the connections, electrode placement, electrode moisture, and batteries.
5. After neuromuscular blocking agents (NMBA) are initiated, TOF should be assessed every hour until the desired level of block is obtained and then re-assessed at least every 4 hours and more often if titration is required. See Table 178-2 for TOF responses indicating the degree of neuromuscular blockade present; adequate paralysis is indicated by one or two twitches. Common clinical practice for patients receiving NMBA and sedation is to use 80 mA for ongoing TOF monitoring.
6. Document TOF response indicating number of twitches out of four (i.e., ¼ for one twitch), the site, and the mA used.
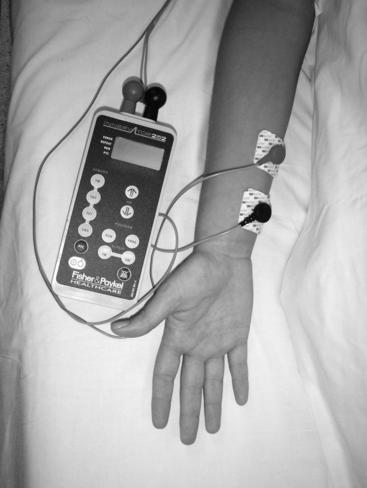
Figure 178-1 Placement of electrodes for TOF testing at the ulnar site.
(Photograph courtesy of P. Howard).
Table 178-2 TRAIN OF FOUR RESPONSE
| Train of Four Response | Approximate Percentage of Receptors Blocked by Agent |
|---|---|
| Four contractions | 0% to 75% |
| Three contractions | 75% |
| Two contractions | 80% |
| One contraction | 90% |
| No contraction | 100% |
Adapted from Jones, S. K. (2003). An algorithm for train-of-four monitoring in patients receiving neuromuscular blockade. Dimensions of Critical Care Nursing, 22(2), 50–57.
PATIENT TEACHING
Describe rationale for use of peripheral nerve stimulation to family members. Explain to families that the muscle contractions (twitches) observed are not painful (Rowlee, 1999).
Jones S.K. An algorithm for train-of-four monitoring in patients receiving neuromuscular blockade. Dimensions of Critical Care Nursing. 2003;22(2):50–57.
Rowlee S.C. Monitoring neuromuscular blockade in the intensive care unit: The peripheral nerve stimulator. Heart & Lung. 1999;28(5):352–362.
University of North Carolina Hospitals (UNC). (2005). Nerve stimulation peripheral. Nursing Procedure Manual (revised September 2005). Retrieved May 16, 2006, from www.unchealthcare.org/site/Nursing/nurspractice/procedures/procedures/proceduren3.pdf/
PROCEDURE 179 Streptokinase Administration
INDICATIONS
To lyse thromboses in the presence of:
1. Acute myocardial infarction (AMI) diagnosed by the following:
2. Pulmonary embolism diagnosed by angiography or lung scan, involving obstruction of blood flow to a lobe or multiple segments, with or without unstable hemodynamics
4. Arterial thrombosis or embolism
CAUTIONS
1. Previous treatment with streptokinase or exposure to Streptococcus infection in the past 6 months (this could result in the formation of antibodies and create resistance to the drug)
2. Recent (within 10 days) major surgery, obstetrical delivery, or organ biopsy
3. Recent (within 10 days) gastrointestinal bleeding
4. Recent (within 10 days) trauma, including cardiopulmonary resuscitation
5. Recent (within 10 days) puncture of subclavian or internal jugular vessel
6. Hypertension: systolic blood pressure greater than 180 mm Hg or diastolic blood pressure greater than 110 mm Hg
7. High likelihood of left-sided heart thrombus (e.g., mitral stenosis with atrial fibrillation)
8. Subacute bacterial endocarditis
9. Hemostatic defects, including those secondary to severe hepatic or renal disease
13. Diabetic hemorrhagic retinopathy
14. Septic thrombophlebitis or occluded arteriovenous cannula at a seriously infected site
15. Current treatment with oral anticoagulants
16. Any other condition in which bleeding constitutes a significant hazard or would be particularly difficult to manage because of its location
EQUIPMENT
PATIENT PREPARATION
1. Perform a thorough assessment, including:
2. Establish continuous cardiac monitoring (see Procedure 55).
3. Establish IV access with a minimum of two to three sites using 18- to 20-G catheters. The use of a double-lumen peripheral IV catheter may reduce the number of punctures (see Procedure 60).
4. Provide routine AMI care as prescribed, which may include but is not limited to the following (Antman et al., 2004):
NOTE: Heparin, if prescribed, will be given after streptokinase administration. See step 7 under Procedural Steps.
5. Administer pretreatment corticosteroids or diphenhydramine as prescribed.
6. Obtain laboratory studies, including the following:
PROCEDURAL STEPS
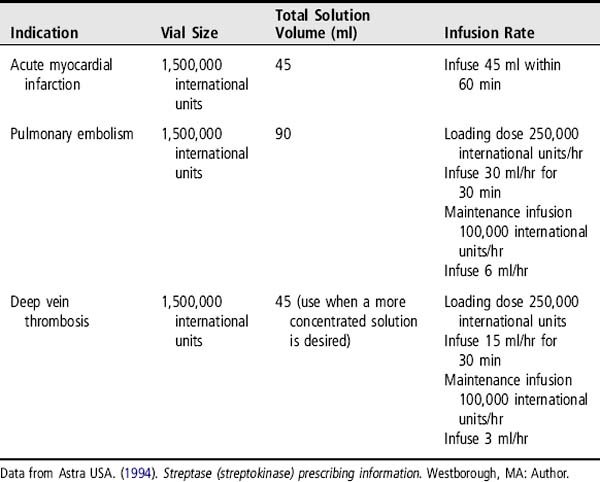
1. Mix streptokinase for administration. In some institutions, the pharmacy prepares the streptokinase solution.
2. When diluting the 1,500,000 international units infusion bottle (50 ml), as in the AMI dose, follow the same procedure as in step 1a, but after the initial reconstitution, the drug can be further diluted in the same vial with an additional 40 ml of NS or D5W.
3. Assemble IV tubing. An in-line IV filter size 0.8 micron or larger may be used.
4. Because streptokinase contains no preservatives, it must be reconstituted just before use. It may be stored up to 8 hours at 2° to 8° C (36° to 46° F) if necessary.
5. Because of the limited availability of compatibility information, no other medications should be added to the container of streptokinase.
6. Administer the loading dose and initiate the maintenance dose as outlined in Table 179-1. Note that there is no loading dose for use with AMI.
7. Anticoagulation with heparin sodium may or may not be required after streptokinase administration (Astra USA, 1994). If heparin sodium is used intravenously, a bolus dose is not administered, and the continuous heparin infusion is begun only after the PTT falls to 2 times the normal control value. This can take several hours to occur after the conclusion of the streptokinase infusion. Therefore, the PTT should be monitored every 2 hours to determine when it falls in that range. Subcutaneous heparin may be administered in lieu of IV heparin (GUSTO, 1993).
PATIENT MANAGEMENT
1. Monitor cardiac rhythm and neurologic status continuously.
2. Monitor patient for signs and symptoms of allergic response, which can vary from a low-grade fever to an anaphylactic reaction. For a mild-to-moderate reaction, the physician may prescribe a corticosteroid and an antihistamine, but the streptokinase infusion may be continued. For a severe allergic reaction, the streptokinase infusion should be stopped and anaphylaxis treatment initiated.
3. Assess and document the vital signs every 5 to 10 minutes during streptokinase infusion. Be alert for a precipitous drop in blood pressure. Decrease the rate of streptokinase infusion if hypotension occurs. Hypotension may require volume replacement, pressor therapy, or both.
4. Monitor and document clinical signs of reperfusion, which include cardiac arrhythmias, resolution of chest pain, and normalization of the ST segments. The arrhythmias seen are the same as those seen in many patients having an MI and may include ventricular tachycardia, ventricular fibrillation, sinus bradycardia, accelerated idioventricular rhythm, and heart block. Any arrhythmias that occur are treated following Advanced Cardiac Life Support (ACLS) guidelines.
5. Assess for signs of bleeding complications and coronary artery reocclusion (e.g., recurrence of chest pain or ST segment elevation) every 15 minutes during the infusion and every 2 hours thereafter until 2 hours after the heparin is discontinued.
6. Document the time the chest pain resolves and the time the streptokinase infusion ends.
7. Monitor the PTT every 2 hours. When the PTT falls to less than two times the normal control, a continuous heparin infusion may be initiated.
8. Institute thrombolytic bleeding precautions, which include the following:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree