The purpose of most clinical laboratory testing is to aid clinical diagnosis and monitor the progress of disease or effectiveness of therapy. By contrast, the purpose of the test that is the subject of this chapter is to prevent disease. The cervical screening test (sometimes referred to as the cervical smear or ‘Pap’ test) involves the microscopical examination of cells recovered by scraping the surface of the cervix. Abnormal changes in the appearance of these cells occur up to 10–15 years before cancer of the cervix develops. Since early treatment of these changes prevents cervical cancer developing, women are actively encouraged to have the test at regular intervals. In the UK around 4.5 million cervical samples are examined in clinical laboratories every year1,2,3. This work accounts for a significant proportion of the total workload of cytopathology departments.
The cervix
Anatomy
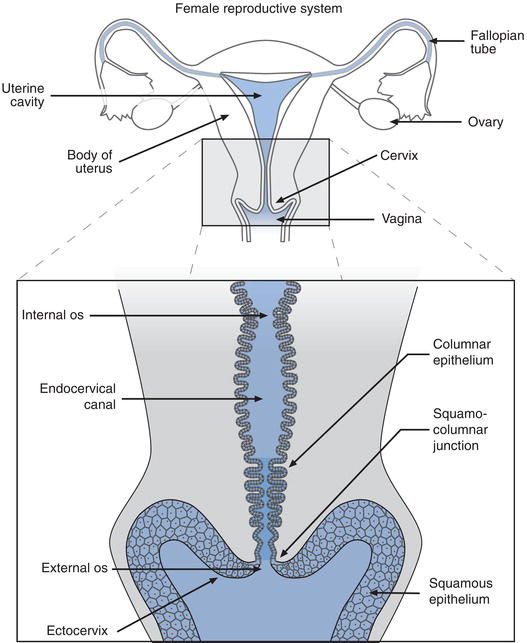
The cervix (from the Greek meaning neck or neck like) is part of the female genital tract. It is a tubular structure around 3 cm in length that forms the lower part or neck of the uterus, connecting the uterus to the vagina. Four main anatomical features can be identified (Figure 24.1). The ectocervix is the lower part of the cervix, which extends into the vaginal canal. At the centre of the ectocervix is the external os, the tiny opening to the endocervical canal, which is circular in women who have never been pregnant but otherwise slit-like. The endocervical canal is the tubular structure of the cervix, which opens at the internal os to the uterus.
As the connection between uterus and vagina, the cervical canal is the first part of the birth canal. Late in pregnancy, the normally tough and fibrous cervix softens or ‘ripens’, allowing the cervical canal to dilate to several times its normal diameter, for passage of the developed foetus from uterus to vagina, during birth.
Cervical epithelium
The surface of the cervix is covered with a protective layer of epithelium. It is the epithelial cells, which make up this protective layer that are sampled for the cervical screening test. In the case of the cervix, there are two sorts of epithelium: squamous epithelium covers the ectocervix, whilst columnar epithelium lines the endocervical canal. Four distinct layers of squamous epithelium can be distinguished on the surface of the ectocervix. The deepest of these is the basal layer, which comprises one row of immature squamous (basal) epithelial cells. Above this is the parabasal layer: two rows of immature squamous (parabasal) cells, which are constantly dividing to maintain the epithelium above. The intermediate layer comprises four to six rows of more mature cells, and the most mature squamous epithelial cells are those within the five to eight rows of the superficial layer. Cells in this superficial layer become increasingly less attached to each other and are continuously cast off from the surface of the ectocervix by a process called desquamation or exfoliation. Constant regeneration of squamous epithelial cells in the basal layers is required to replace those that are lost from the surface of the ectocervix by exfoliation. Most of the squamous epithelial cells recovered for the cervical screening test are from the superficial and intermediate layers. Cells from the parabasal layer constitute only around 5% of all squamous epithelial cells in a normal sample from young women. The cells in a cervical sample from older women contain slightly more parabasal cells, and disease of the cervix is associated with a significant increase in the number of parabasal cells in a cervical sample. Because of their relative depth, basal cells are rarely seen in cervical samples.
The mucous secreting columnar epithelium of the endocervical canal is composed simply of one row of columnar epithelial cells; some are mucous secreting and others have cilia on their surface. The mucous and cilia are thought to facilitate the passage of spermatozoa through the endocervical canal. When columnar epithelial cells are seen in a cervical sample, they are referred to as endocervical cells.
The squamo-columnar junction and transformation zone
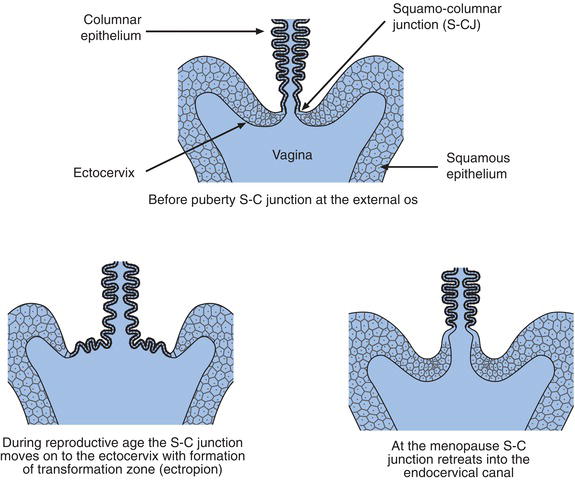
The point where the squamous epithelium of the ectocervix meets the columnar epithelium of the endocervical canal is called the squamo-columnar junction (Figure 24.1). This junction is of great pathological significance because it is in this area that most cases of cervical cancer originate. Before puberty the junction lies at the external os, (Figure 24.2) but in response to the normal hormonal changes that occur at puberty, the lower end of the endocervix everts somewhat, so that the junction moves outwards from external os onto the ectocervix. A second important physiological change occurs as a result of this eversion of the endocervix. The columnar epithelium that covers the everted part of the endocervix is now exposed to the acidic vaginal environment and this environmental change, in combination with other factors, induces the exposed columnar epithelial cells to undergo transformation to squamous epithelial cells, a process called metaplasia. The area around the external os of the ectocervix where this transformation of columnar to squamous epithelium occurs is called the transformation zone. Epithelial cells undergoing transformation are called squamous metaplastic cells. As the transformation occurs, throughout a woman’s reproductive years, the squamo-columnar junction moves back towards the external os. After the menopause the junction usually retreats into the endocervical canal.
Cancer of the cervix
Almost all (85–90%) cervical cancers originate in the squamous epithelial cells of the transformation zone on the ectocervix. This is known technically as cervical squamous cell carcinoma. The rest originate in the columnar epithelial cells of the endocervix; this is known as cervical adenocarcinoma. Generally speaking the second of these two kinds of cervical cancer is considered to have the worst prognosis.
With an annual incidence of around 2 900 in the UK, cervical cancer is currently the twelth most common cancer to affect women of all ages, and the second most common cancer to affect young women under the age of 35 years4. It can affect women of any age but is most often diagnosed between the ages of 25 and 50 years. Younger women, so long as they are of reproductive age, are also at risk; around 2% of cases occur before the age of 255.
There are often no symptoms during the early stages of cervical cancer. The only common symptoms are abnormal vaginal bleeding and discomfort during sexual intercourse. Post-coital bleeding is fairly common.
The prognosis for a woman with invasive cancer of the cervix depends, as with most other cancers, on the extent of cancer spread at the time of diagnosis. So long as the cancer is confined to the cervix, the prognosis is good; treatment can effect a cure. Untreated, invasive cervical cancer spreads from the cervix first to the upper part of the vagina, then to the ureters and lower part of the vagina. In the most advanced cases, invasion of the bladder wall and rectum may be evident at diagnosis. Such advanced disease is associated with a poor prognosis; only 15–20% of patients with the most advanced disease survive more than five years. The annual UK death toll due to cervical cancer is currently close to 9504. There is now overwhelming evidence that infection with the human papilloma virus (HPV) is the cause of cervical cancer.
HPV and cervical cancer
Early epidemiological studies suggested that a sexually transmitted agent might have a role in cervical cancer. By the end of the 1970s human papilloma virus (HPV) had emerged as the most likely of several candidate sexually transmitted agents. The more recent observation that HPV is present in all cervical cancers and that infection predates cancer development has allowed the now established view that HPV is a necessary, but not sufficient, cause for cervical cancer6. Thus only those who have been infected with HPV develop cervical cancer. (It is worth mentioning in passing that since the link between HPV and cervical cancer was first established it has become apparent that some other cancers are caused by HPV infection; these include almost all anal cancers, and some vaginal, vulvar, penile and mouth/throat cancers.)
Cervical cancer is by no means an inevitable consequence of HPV infection. Up to 75% of sexually active women become infected with HPV at some point in their life7. The vast majority eradiacte the virus without even knowing they have been infected. It remains unclear why in a small proportion of infected women HPV is not eradicated but persists for many years, eventually causing cervical cancer. Some risk factors have been identified; they include cigarette smoking, the use of oral contraceptives and immune suppression (e.g. co-infection with the human immunodeficiency virus (HIV) that causes AIDS).
There are around 100 different HPV types, which have been broadly categorised to those that infect skin (the cutaneous types) and those that infect the mucosal surface of the mouth and genital tract (mucosotropic types). The cutaneous HPV types are responsible for common, invariably benign, wart infection of hands and feet. The mucosotropic group includes 40 different HPV types that, following transmission by sexual contact, infect the genital tract. These are divided into low risk types that can cause benign genital warts and high-risk types that can, after many years of clinical latency, cause cervical cancer. Seventeen high-risk types have been identified but just five types (HPV 16, 18, 31, 33 and 45) account for 95% of all cervical cancers. The two most common high-risk types, HPV 16 and HPV 18, account for an estimated 70–75% of cases.
The notion that prevention of HPV infection prevents cervical cancer provided the rationale for universal HPV vaccination of girls before they become sexually active, and thereby at risk of HPV infection.
The UK HPV vaccination programme, introduced in 2008, offers vaccination (delivered in three doses) to all girls aged 12–13 years. Initially, the vaccine used was Cervarix, which provides protection against HPV types 16 and 18. Since 2012 an alternative vaccine, Gardisil, has been used. This provides the same level of protection against HPV types 16 and 18, but also provides protection against HPV types 6 and 11 that together cause almost all cases of genital warts. If vaccination achieves 80% coverage it is estimated that by 2025 there will be a 63% reduction in the incidence of cervical cancer; most recent data8 indicate that 83% of girls are being vaccinated. Since vaccination provides full protection only against HPV types 16 and 18 infection, and other HPV types can cause cervical cancer, it is important that vaccinated women continue to participate in the screening process.
The malignant (cancerous) potential of HPV depends on its DNA being incorporated into the DNA of normal healthy cervical cells. All pre-cancerous and cancerous cervical cells contain HPV DNA. The notion that HPV DNA testing of cervical samples would be of value in screening for cervical cancer has, after many years of research, been established, and this test was introduced to the screening process in 2011. Just how this testing is applied will be discussed further.
Natural history of cervical cancer
The preventative value of the cervical screening test is due to the usually long natural history of cervical cancer. Identifiable pre-cancerous changes occur up to 10–15 years before invasive cancer develops. If these are identified and treated, cervical cancer can be prevented.
Many years before cervical cancer develops, microscopical changes to the squamous epithelial cells in the transformation zone occur. These changes are known technically as cervical intra-epithelial neoplasia (CIN). CIN is a potentially progressive lesion, caused by HPV infection, which may if not treated ultimately lead to cervical cancer. Three grades of severity of CIN have been identified. If the cellular changes which characterise CIN are confined to the lowest third of epithelium, a diagnosis of CIN I is made, if such changes are seen in the lower two thirds, CIN II is diagnosed. The most severe form of CIN, CIN III, is diagnosed when abnormal cells are seen throughout the full thickness of the epithelium; this is also called carcinoma in situ. The level of CIN determines the risk of invasive cervical cancer developing. For example, patients with CIN I have a relatively low risk of cancer developing; in around 50% of cases the abnormality resolves spontaneously. However CIN I may persist without any untoward effects; or for an unpredictable small minority of patients may progress through CIN II to CIN III. Around 30% of patients with CIN III if left untreated would progress to invasive cancer within ten years. At the present time there is no way of predicting with any certainty which patients with CIN will progress to invasive cancer, or how speedy that progression will be. All that can be said is that there is an increased risk of cervical cancer for women with CIN and that the risk is highest in those with CIN III. Treatment of CIN prevents cancer developing in nearly all cases. The definitive diagnosis of CIN depends on microscopical examination of a piece of cervical tissue (a biopsy). However the microscopical appearance of cells scraped from the surface of the cervix reflect the abnormalities that constitute CIN. This is the rationale for the cervical smear test as a screening test for prevention of cervical cancer.
Cervical screening
History
The cervical screening test is often referred to as the ‘Pap’ smear test. This alternative name celebrates the research of George Papanicolaou, a Greek physician who worked in the United States. In the 1920s Papanicolaou first observed that cancerous cervical cells could be found in vaginal smears. To enhance the appearance of these cells he developed a staining technique using the Papanicolaou stain, which is used to this day to stain cervical samples. In the late 1940s, Ayre demonstrated that scraping the surface of the cervix was a more reliable means of recovering cancerous cervical cells. He developed the Ayre’s spatula for this purpose. At around this time, the concept of pre-cancerous disease of the cervix was introduced, allowing the rationale for the use of the cervical smear test to screen for early evidence of cervical cancer.
A screening programme using the cervical smear test was first introduced in the UK during 1964. However the organisation of the scheme was not nationally co-ordinated and evolved in an ad hoc fashion. The result was that the scheme had much less impact in reducing the incidence of cervical cancer than expected. Experience of cervical screening in other European countries, particularly Denmark, Sweden and Finland, had demonstrated that a well organised scheme in which all women at risk are regularly given a cervical smear test can be successful. In these countries there had been huge steady reduction in the incidence of cervical cancer from the time screening was introduced in the 1960s. In recognition of the relative failure of the ad hoc screening programme then in operation in the UK, a nationally co-ordinated cervical screening programme was introduced in 1988. Although there have been changes over the intervening years, the scheme introduced in 1988 remains substantially the same.
Probably the most significant change to the scheme came during the period 2005–2008 when the switch from conventional cervical smear test to liquid based cytology (LBC) occurred in stages across the country. Instead of smearing the sample on a glass slide and sending this to the laboratory, the LBC technique involves collection of the cervical sample into a liquid fixative solution that is sent to the laboratory. This apparently simple change has made the screening process far more efficient and cost effective, principally because the LBC method is associated with a much lower proportion of samples being reported as inadequate, and having to be repeated. Prior to introduction of LBC around 10% of cervical screening tests had to be repeated because of inadequate sampling; now only around 2.5% of tests need repeating9.
LBC has also allowed much speedier reporting of results. Recent data shows that results of all cervical screening tests are now available within one to two weeks of the sample being taken10. Prior to implementation of LBC women had to wait 4–12 weeks for their result, and sometimes even longer. The conventional smear technique only allowed cytological examination of cervical samples, whereas the LBC technique allows both cytological examination and HPV DNA testing. Introduction of selective HPV DNA testing in 2011–2012 represents the most recent improving change to the cervical screening programme.
Organisation and success of cervical screening
The aim of the UK programme is to reduce the incidence of cervical cancer and deaths due to cervical cancer by performing regular cervical screening tests on every woman between the ages 25 and 65 years. Current national policy in England and Northern Ireland is that all women between the ages 25 and 49 should be screened every three years, and those between the ages 50 and 65 every five years. Screening after the age of 65 is reserved for those who have not been screened since the age of 50 and those who have had recent abnormal results. In Scotland and Wales the policy is to screen all women between the ages 20 and 65 (60 in Scotland) every three years, but there is now consultation on the proposal to change this to that which currently pertains in England and Northern Ireland; a decision is expected by 2013.
The responsibility for cervical screening falls largely on the primary healthcare team. A computerised call and recall system implemented by primary care workers (GPs, practice nurses and practice managers) ensures that every woman in the target population of each GP practice is offered a cervical screening test appointment at the prescribed intervals.
Before 1987 only around 40% of women in the then target age range 20–65 years were being screened. With the introduction of the national screening programme in 1988, coverage increased quickly. By 1994, 85% of the target population was being screened; this level of coverage has been maintained broadly ever since, although there is concern that coverage has reduced in recent years, particularly among young women when offered their first screening appointment. In 2010 78.6% of the total target population attended for cervical screening10.
Notwithstanding this recent concern, there is much evidence that the increased level of cervical screening since 1987 has had a significant effect on incidence of cervical cancer11. In England, between 1971 and 1987 the annual incidence stayed fairly steady, fluctuating between 14 and 16 per 100 000 women (i.e. on average 3900 cases a year). Between 1990 and 2003 however, annual incidence fell steadily year on year; by 1995 the incidence was 10 per 100 000 women or 2900 new cases, and in 2003 just 2312 new cases were registered12. Since 2003 annual incidence in England has remained fairly static at around 2300.
The screening programme since 1987 has also had an effect on the number of deaths attributed to cervical cancer. From 1950 to 1987 mortality due to cervical cancer fell steadily at the rate of 1.5% every year. Since 1987 this rate of fall has trebled. In 1987, 1800 deaths in England were attributed to cervical cancer; in 2008 this number had fallen to 759. An analysis13 of trends in mortality before screening was introduced suggests that cervical screening prevents the death of 5000 women in the UK every year.
The cervical screening test
Around 80% of cervical samples are collected in a primary care setting, most often by practice nurses.
Patient preparation
The best time to take a cervical sample is mid cycle to avoid contamination with menstrual blood. It is preferable to delay the test for a few months following childbirth. The patient should be advised to avoid the use of vaginal creams and refrain from sexual intercourse for 24 hours before the test. Many women will be anxious, especially on the first occasion they attend, so that a calm reassuring manner is important. A brief explanation of the test emphasising the points in Table 24.1 will help to allay fears. Any effort made to reduce the tension or anxiety a woman might experience at the time of sampling, will increase the chance of obtaining a suitable sample. Furthermore there is evidence to suggest that women who are dealt with in a sympathetic manner are more likely to re-attend for future testing or further investigation, if an abnormality is discovered.
Cervical sampling technique
The practical detail of collecting an adequate cervical sample is beyond the scope of this chapter. Some general points are made here.
The cervix must first be visualised by passing a vaginal speculum. The cervix must be well illuminated. The object is to sample epithelial cells from the transformation zone and squamo-columnar junction, so that squamous epithelial cells as well as some endocervical cells are recovered. Since the position of the squamo-columnar junction varies with age and parity, sampling technique must take account of these factors.
A disposable sampling instrument called a Cervex-brush is used, the business end of which is a soft flexible plastic brush. The shape of the brush with longest bristles at the centre reflects the contours of the ectocervix and endocervix, allowing the brush to come into contact with all areas of the transformation zone, including the squamo-columnar junction. The full circumference of the transformation zone must be sampled by applying slight pressure on the cervix with the Cervex-brush and rotating it clockwise through 360o several times. Cervical cells adhere to the brush.
Table 24.1 Topics for discussion with patient prior to cervical smear test.
Why have the test?
About the test
After the test
|
A differently designed brush that allows sampling within the endocervical canal is necessary in the case of post-menopausal women because the transformation zone retreats within the endocervical canal at the menopause.
The brush, whatever its design, is immediately transferred to a glass vial containing fluid fixative, and the sampled cellular material is rinsed from the brush by a swirling motion. Alternatively the head of the brush is removed, placed in the liquid fixative vial and the vial is shaken vigorously to release cellular material. The vial is transported to the laboratory.
In the laboratory cervical cells are separated from other debris in the cervical liquid sample and these cells are transferred in an even distribution to a glass slide, prior to staining and microscopical examination by a cytoscreener.
Results of cervical screening test
Around 93–94% of adequately collected cervical samples are found on microscopical examination to be entirely normal9 and no further investigation, save recall in three or five years is necessary. The remaining 6–7% have some degree of abnormality ranging from the benign (the vast majority), through entirely curable pre-malignant disease (CIN) to invasive cancer.
The main object of microscopical examination of cervical cells is to search for the abnormal changes associated with the pre-cancerous condition, CIN. These abnormal changes, which are known collectively as dyskaryosis (literally, abnormal nucleus), include an increase in the size of the nucleus compared with surrounding cytoplasm, along with irregularity in the shape and staining characteristics of the cell nucleus. There are three recognised grades of severity of dyskaryosis: mild, moderate and severe. In some cases only slight changes to the nucleus are present which may not be sufficient to warrant a report of even mild dyskaryosis; these are reported as ‘borderline nuclear abnormalities’. The severity of dyskaryosis correlates to some degree with the level of CIN that might be expected if a cervical tissue biopsy were examined, so that a report of a smear result from a patient with dyskaryosis implies a prediction of the level of CIN. In broad terms CIN I would be expected if mild dyskaryosis were present; CIN II or CIN III would be expected if moderate dyskaryosis were present and CIN III would be expected if severe dyskaryosis were present. It is not possible to make a definitive diagnosis of CIN or invasive cervical cancer from examination of a cervical smear.
Follow up of abnormal samples – HPV testing
The cytological examination of cervical cells described above is only a screening test; its value lies in its ability to exclude the approximate 93% of women whose cervical cells appear normal. The severity of dyskaryosis found in an abnormal sample merely determines the next step in the diagnostic process. The vast majority (around 90%) of abnormal samples show only the mildest of abnormality, reported as either ‘borderline nuclear abnormalities’ or ‘mild dyskaryosis’. There is a strong likelihood that these mild changes will spontaneously regress to normal. However there remains a risk that over time a pre-malignant lesion might develop. Prior to introduction of HPV testing, those with these mild abnormalities would have been advised to attend for repeat testing at three or six monthly intervals until the abnormality had resolved. Now, their samples are recovered and tested for the presence of HPV DNA. If the test is negative (i.e. cervical cells contain no HPV DNA) there is no risk of pre-malignancy and patients are able to return to routine screening every three or five years. Those with mild cell abnormality (i.e. ‘borderline nuclear abnormalities’ or ‘mild dyskaryosis’) and a positive HPV DNA result however are referred for colposcopy, as are all patients whose smear show signs of ‘moderate’ or ‘severe’ dyskaryosis.
Colposcopy
Colposcopy is a usually pain free, outpatient diagnostic procedure in which the cervix is viewed directly through a specially modified microscope, called a colposcope. A vaginal speculum is passed as for a cervical smear, and the cervix is ‘painted’ with acetic acid and a stain which both help to visualise abnormal tissue. This is biopsied to determine the level of CIN or confirm the presence of micro-invasive or invasive cancer. The results of colposcopy and cervical tissue biopsy determine the treatment that may be offered. In the absence of invasive cancer, CIN can be treated in an outpatient setting using a colposcope, by a variety of techniques which all involve the destruction (ablation) of abnormal tissue by extremes of heat (e.g. laser vaporisation, cryotherapy, loop diathermy). Surgical excision (cone biopsy) may be necessary, and rarely, in the absence of invasive disease, hysterectomy might be recommended. All patients who have been treated for CIN must be monitored for recurrence of disease. Instead of the normal three or five year interval between cervical screening test, such patients may be recalled every year for up to ten years. HPV testing is used in this follow up – a negative result on both cytological examination and HPV testing is evidence of cure, and an indication to return to normal interval cervical screening.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree