- ABO and Rh blood group systems
- Incompatible transfusion reaction
- Other risks of transfusion
- Safe transfusion practice
- Antibody screening during pregnancy
- Haemolytic disease of the newborn
Every year in the UK over 2.5 million units of donated red cells are transfused to hospital patients. Transfusion is such a commonplace procedure that it is perhaps easy to underestimate the dangers involved. Although often of life-saving benefit, red cell transfusion is associated with considerable potential risk to the recipient patient. The two most significant risks are: transmission of serious blood born infection and the potentially fatal haemolytic transfusion reaction that can occur if patients receive incompatible red cells. The risk of infection is virtually eliminated1 by careful donor selection and rigorous screening of all blood donations for evidence of infection (Table 20.1). In the UK, this screening procedure, which ensures supply of the safest possible blood products to local hospital blood transfusion laboratories, is the responsibility of the four national blood transfusion services. Prevention of the second major risk associated with red cell transfusion, incompatible transfusion reaction, begins with the pre-transfusion tests of donor and recipient blood conducted in local hospital blood transfusion laboratories. Of crucial importance are the three tests that are the subject of this chapter: determination of blood group, antibody screen and crossmatch. In order to understand what is meant by an incompatible red cell transfusion and the significance of these tests for its prevention, a little background immunology is required.
Table 20.1 Some of the selection criteria for blood donors and mandatory tests on donated blood to prevent transmission of infectious disease.
| Blood donors must: |
| be aged between 17 and 65 years be in good health weigh more than 50 kg have a haemoglobin (Hb) greater than 13.5 g/dl if male and greater than 12.5 g/dl if female |
| Blood donors must not: |
| be pregnant or have been pregnant during previous 12 months have ever suffered from cancer, syphilis or brucellosis have a recent history of malaria, hepatitis jaundice or glandular fever be in a high risk group for HIV infection have received a blood product transfusion since 1980 donate blood more than three times a year |
| All donated blood is tested for the presence of: |
| hepatitis B surface antigen antibody to hepatitis C hepatitis C nucleic acid antibody to Treponema pallidum |
Background immunology
What are antigens and antibodies
Our ability to withstand attack from invading micro-organisms, such as bacteria and viruses, depends in part on antibody produced by plasma cells, derived from white blood cells called B-lymphocytes. Antibodies are immunoglobulin proteins. They bind to and neutralise bacteria and viruses. Each antibody is very specific in its action, so that an antibody that binds and neutralises one sort of bacteria will have no effect on another. This specificity is due to the specific nature of the molecular target on the surface of each sort of bacteria. This molecular target is called the antigen. Recognition by B-lymphocytes of specific bacterial or viral antigens induces specific antibody production.
This ability of the body to produce destructive antibodies to ‘foreign’ antigens is not confined to those antigens present on the surface of bacteria or viruses. Proteins and other molecular substances present on the surface of any foreign (i.e. non-self) cell are ‘seen’ as antigens and provoke a similarly destructive specific antibody response. It is, for example, this same antibody response to foreign antigens that accounts in part for the tissue rejection that can occur following organ transplantation.
To summarise then:
An antigen is any substance (most commonly a protein, but may be a carbohydrate) that causes production of antibodies by plasma cells (B-lymphocytes). Antigens are found usually, although not exclusively, on the surface of cells.
An antibody is a protein (immunoglobulin) which circulates in blood plasma and binds only with the antigen that provoked its production.
When an antibody binds with an antigen present on the surface of a cell, be it a bacteria, virus or tissue cell, a sequence of events follows which invariably leads to that cell’s disruption or destruction.
One of the characteristics of the immune system is its ability to remember. On the first occasion lymphocytes ‘meet’ a foreign antigen, antibody production is low and therefore not very effective. However the immune system is now primed. Specialised lymphocytes (called memory lymphocytes) ‘remember’ the antigen, so that when it is encountered on subsequent occasions, both speed and intensity of antibody production are greatly increased. This is the basis of the concept of acquired immunity: we have limited protection (immunity) against a particular bacteria or virus until our immune system has been primed by initial exposure.
Clearly it is vital for health that we do not produce antibodies to our own antigens, and the immune system has the means to prevent this occurring. However it is worth mentioning in passing that there are a large group of pathological conditions, collectively known as the autoimmune diseases, in which this ability to distinguish ‘self’ from ‘non-self’ antigens is lost. These diseases are characterised by the production of antibodies (autoantibodies) directed at ‘self’ antigens. Common diseases with an autoimmune component include rheumatoid arthritis, Type 1 diabetes, most thyroid diseases and systemic lupus erythematosus (SLE); there are many others.
Notwithstanding these pathological exceptions, it is important to remember for the discussion here that it is normally not possible to produce antibodies to one’s own antigens.
Red cell antigens and blood group
The surface of red cells like all other cells are covered with inherited antigens. These red cell antigens (or the lack of them) determine an individual’s blood group. More than 700 different red cell antigens (most very rare) have been identified; these make up the 30 blood group systems so far described. Fortunately, only a tiny minority of these antigens are of significance in transfusion medicine. Of those that do have significance, the antigens of the ABO and Rh blood group systems are of prime importance.
ABO blood group system
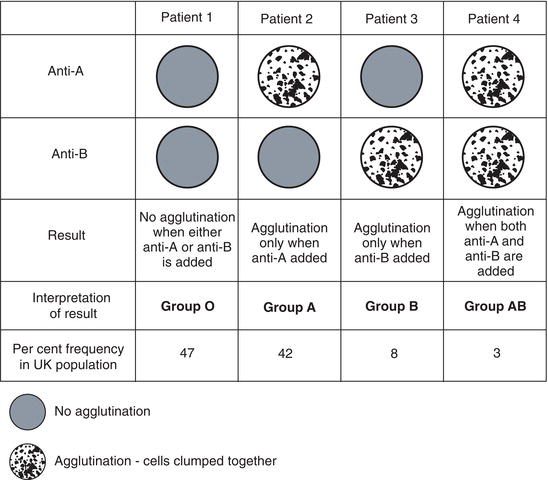
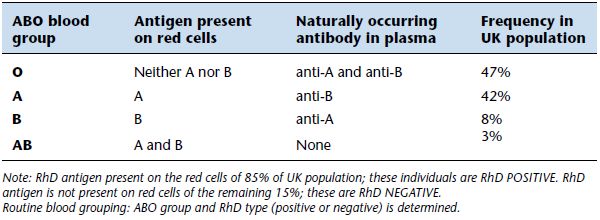
We all belong to one of four groups of the ABO blood group system determined by the inheritance or non-inheritance of two red cell antigens, A and B. Those who inherit neither A nor B red cell antigens belong to group O; those who inherit the A red cell antigen belong to group A, those who inherit the B red cell antigen belong to group B and those inherit both the A and the B red cell antigen belong to group AB. Most (88%) of the UK population belong to either group O or group A.
Rh blood group system
The Rh blood group system, so called because early research was conducted on rhesus (Rh) monkeys, is the only other blood group system of major significance to blood transfusion. There are five red cell antigens in the Rh system – C, c, D, E and e – but only the D antigen is of major significance. Around 85% of UK population have the D antigen on their red cells and are said to be Rh D positive, the remaining 15% do not have the D antigen and are Rh D negative.
Other less significant red cell antigens
A routine blood group means determination of patient ABO group type and Rh D type (either positive or negative). Of the remaining 400 plus red cell antigens, which may or may not be present on the surface of an individual’s red cells, a few have occasional significance for transfusion medicine. Among these are other antigens of the Rh blood group system (i.e. the c, C, E and e antigens) and antigens of the Kell (K), Duffy (Fy), Kidd (Jk), MNS and Lewis (Le) blood group systems. These antigens are rare causes of transfusion reaction either because they are themselves rare or because they are relatively weakly immunogenic.
Antibodies to red cell antigens: incompatible blood transfusion
The significance of red cell antigens for blood transfusion medicine lies in the specific antibodies to these red cell antigens, which may or may not be present in the recipient’s plasma. An incompatible transfusion reaction occurs when antibody present in the patient’s (recipient) plasma binds to its complementary antigen present on the surface of (donor) transfused red cells. Such antibody–antigen binding can result in the destruction of the donated red cells; this destruction is called haemolysis so that the term immune haemolytic transfusion reaction is used to describe this adverse effect of blood transfusion. So long as the patient’s plasma contains no significant antibodies to the antigens present on the red cells of donated blood, the patient and donor blood are said to be compatible and donor blood can be safely transfused.
Production of red cell antibodies
It was stated above that antibodies are only produced when B-lymphocytes come into contact with the relevant ‘foreign’ antigen. There are two situations in which an individual’s antibody producing lymphocytes may come into contact with ‘foreign’ red cell antigens. The first of course is blood transfusion, and the second is pregnancy. During labour of pregnancy, and sometimes earlier in pregnancy, foetal blood leaks to maternal circulation. The volume of this so called fetomaternal haemorrhage is usually < 0.1 ml but can, in a small minority of pregnancies, be as much as 30 ml. If foetal red blood cells bear antigens inherited from the father, which are not present on the mother’s red cells, they are ‘seen’ as foreign by the mother’s lymphocytes, which then proceed to manufacture antibody.
As with any other immune response, initial antibody production during the first immunising blood transfusion or pregnancy is low and usually has no effect. But the immune system is now primed to synthesise large quantities of antibody, the next time the ‘foreign’ red cell antigen is encountered. Antibodies produced in this way are called immune red cell antibodies. The clinically most significant immune red cell antibody is anti-D, the antibody to the Rh D antigen. Of course only those who lack the Rh D antigen (i.e. the 15% of the population who are Rh D negative) can be immunised to produce anti-D in this way. In fact around 1% of the population have anti-D in their plasma, all the result of previous immunising transfusion or pregnancy. If such people were transfused with Rh D positive blood, the anti-D in their plasma would bind to the D antigen on the surface of donated red cells, resulting in a haemolytic transfusion reaction.
Table 20.2 The ABO blood group system and RhD type.
If immune red cell antibodies were the only red cell antibodies present in plasma, then only those who have a history of previous blood transfusion or pregnancy would be at risk of incompatible blood transfusion; this is not the case.
The overriding clinical significance of the ABO blood group system lies in the fact that antibodies to ABO antigens are naturally occurring, that is they do not arise as a result of immunisation by ‘foreign’ red cells. All of us have antibodies to the A or B antigen that we lack. Thus all those who belong to group O and lack both the A and B antigen have antibodies to both antigens (i.e. anti-A and anti-B) in their plasma; all those who belong to group A and have the A antigen on their red cells have antibodies to the B antigen (i.e. anti-B) in their plasma and all those of group B have the antibody to the A antigen (i.e. anti-A) in their plasma. Only the 3% of the UK population belonging to group AB, who have both the A and B antigen on their red cells, have no naturally occurring anti-A or anti-B in their plasma. The antigens and antibodies associated with the four groups of the ABO blood group system are summarised in Table 20.2.
It is the relative ubiquity and potency of naturally occurring anti-A and anti-B that determines the prime clinical importance of the ABO blood group system. Like anti-D, practically all other clinically significant red cell antibodies are immune antibodies so they cannot be present in the plasma of a person who has not been immunised by a previous transfusion or pregnancy. Thus the only significant red cell antibodies present in an individual who has never received a blood transfusion or been pregnant is naturally occurring anti-A or anti-B. It has been estimated that if only ABO compatibility were ensured and no other tests were performed red cell transfusion would be immunologically safe in 97% of cases.
Immune haemolytic transfusion reaction – ABO incompatibility
The consequences of transfusing ABO incompatible blood are described in Figure 20.1. Such a reaction can occur after only a few millilitres of blood have been transfused, such is the potency of anti-A and anti-B. In this example, blood from a donor who is blood group A is transfused to a patient who is blood group B. Anti-A present in the patient’s plasma binds to the A antigen on the surface of donated red cells. The red cells clump together (agglutinate). Antigen-antibody binding activates the so called complement pathway; it is the complement proteins (by convention denoted by the letter C followed by a number), produced as a result of this activation, which accounts for many of the signs and symptoms of a haemolytic transfusion reaction. Complement proteins C5, C6, C7, C8 and C9 are all involved in the process of red cell destruction (haemolysis), in which holes are made through the red cell membrane. By this complement mediated haemolysis, all donated red cells are destroyed in the most severe cases of ABO incompatibility. Complement proteins C3a and C5a initiate an inflammatory response that includes release from activated mast cells of various potent chemicals (e.g. histamine, bradykinin) that causes a sudden fall in blood pressure (hypotension) and other very visible symptoms. The fall in blood pressure leads to symptoms of clinical shock and reduced flow of blood to the kidneys with the onset of acute kidney disease (renal failure) in the most severe cases. Other complications of severe haemolytic transfusion reaction include disseminated intravascular coagulation, a condition discussed briefly in Chapter 17. The excess Hb released from damaged red cells is metabolised to bilirubin, causing jaundice.
Other immune haemolytic transfusion reactions
Even if donated blood is ABO compatible with recipients blood, there remains a risk of immune haemolytic transfusion reaction if there are other significant red cell antibodies present in the patients plasma. Since these are all immune antibodies, they can only be present in the plasma of those patients who have been immunised by previous blood transfusion or pregnancy. The most important of these is anti-D. Others include anti-C, anti-c, anti-E and anti-e (i.e. remaining antibodies to Rh blood group antigens); anti-K (antibody to the Kell (K) blood group antigen); anti-Fya and anti-Fyb (antibodies to two of the Duffy (Fy) blood group antigens); and anti-Jka and anti-Jkb (antibodies to two of the Kidd (Jk) blood group antigens). Symptoms of haemolytic transfusion reaction which result from these antibodies are generally speaking less severe than those associated with ABO incompatibility. The reaction may be delayed for up to ten days after the transfusion, when chills and fever develop. Red cell destruction may cause anaemia and mild jaundice.
Laboratory testing: blood group, antibody screen and crossmatch
Sample collection
When a patient requires a blood transfusion, 7.5 ml of venous blood must be collected into a plain tube containing no additives, or a bottle containing the anticoagulant EDTA, depending on local policy. Blood transfusion laboratories supply designated tubes (usually pink top) that are to be used only for these tests.
Because of the potentially fatal consequences of giving donated blood product to the ‘wrong’ patient, scrupulous attention to the detail of patient identification and documentation is vital when collecting blood for all of these tests. Before taking blood ensure beyond any doubt the identification of the patient, by asking the patient his or her name and cross-checking with his or her identification armband.
The following minimum information must be written legibly on the sample label and accompanying request card:
- Patient’s first and last name (taken from the patient’s armband).
- Hospital number (taken from the patient’s armband).
- Patient’s ward or department.
- Date and time of collection.
- Initials of person taking the blood.
The request card should include the following additional details:
- The nature of donated blood required (whole blood, packed red cells etc.).
- Number of donated units required.
- When the blood is required – level of urgency.
- Why the blood is required (acute blood loss, chronic anaemia, elective surgery etc.).
- ABO and rhesus blood group (if known).
- Any relevant transfusion or obstetric history (if known).
- Signature of the medical officer making the request.
These are guidelines only; healthcare workers involved in the collection of blood for pre-transfusion testing must adhere scrupulously to protocol contained in the local blood transfusion policy document, which should reflect best practice as defined by the British Committee for Standards in Haematology (BSCH) in collaboration with the Royal College of Nursing and the Royal College of Surgeons2,3.
Principles of the three tests
The purpose of the three tests is to prevent immune haemolytic transfusion reaction by issuing only donated red cells that are immunologically compatible with the blood of the patient who is to receive the transfusion. In essence this means first determining which antibodies to red cell antigens are present in the patient’s plasma and then selecting donated blood (red cells) that contain none of the relevant red cell antigens. The only significant antibodies that can be expected with certainty to be present in patient’s plasma are the naturally occurring antibodies to the red cell antigens of the ABO group system, so that determination of the patient’s ABO blood group is the prime test. At the same time as the ABO group is determined the Rh D status (either negative or positive) is also determined. Together these are the routinely determined ‘blood group’ or ‘blood type’.
The binding of red cell antibody to its complementary antigen results in agglutination (clumping together) of cells. This visible phenomenon is exploited in all blood banking tests and is reflected in the alternative name for red cell antibodies and antigens. In many texts red cell antibodies are referred to as agglutinins and antigens as agglutinogens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree