Acute respiratory distress syndrome (ARDS) is a potentially life-threatening syndrome characterized by dyspnea, severe hypoxemias, decreased lung compliance, and diffuse, bilateral pulmonary infiltrates without evidence of left ventricular dysfunction (Hudson et al., 1995). ARDS is acute in onset, frequently developing within 4 to 48 hours, and may persist from days to weeks (Hudson et al., 1995). It can be associated with myriad clinical disorders, including pneumonia, sepsis, aspiration of gastric contents, major trauma, and multiple transfusions of blood products (Sanchez & Toy, 2005; Ware & Matthay, 2000). ARDS can be subclassified as direct or indirect injury to the lungs (Sanchez & Toy, 2005; Ware & Matthay, 2000) (Table 2-1).
| Direct Lung Injury | Indirect Lung Injury |
|---|---|
| Common Causes | Common Causes |
• Pneumonia • Aspiration of gastric contents | • Sepsis • Severe trauma with shock and multiple transfusions |
| Less Common Causes | Less Common Causes |
• Pulmonary contusion • Fat emboli • Near-drowning • Inhalation injury • Reperfusion pulmonary edema after lung transplantation or pulmonary embolectomy | • Cardiopulmonary bypass • Drug overdose • Acute pancreatitis • Transfusions of blood products |
Our understanding of the pathophysiologic processes of ARDS has evolved since the early 1960s. ARDS now is understood to be a constellation of pathologic changes characterized by three stages of diffuse alveolar damage (DAD): the exudative phase, the fibroproliferative phase, and the fibrotic phase (Weinacker & Vaszar, 2001; Tomashefski, 2000). However, the extent of ARDS development and the pathogenesis may vary, depending on the different risk factors.
Exudative Phase
Two distinct barriers together make up the alveolar-capillary barrier: the endothelium and the epithelium (Fig. 2.1). The normal alveolar epithelium is composed of two types of cells: flat type I cells, which make up approximately 90% of the alveolar surface area, and cuboidal type II cells, which make up approximately 10% of the alveolar surface area (Ware & Matthay, 2000). These cells are integral to maintenance of the milieu within the lungs. Their functions include surfactant production, ion transport, and proliferation and differentiation after injury (Tomashefski, 2000).
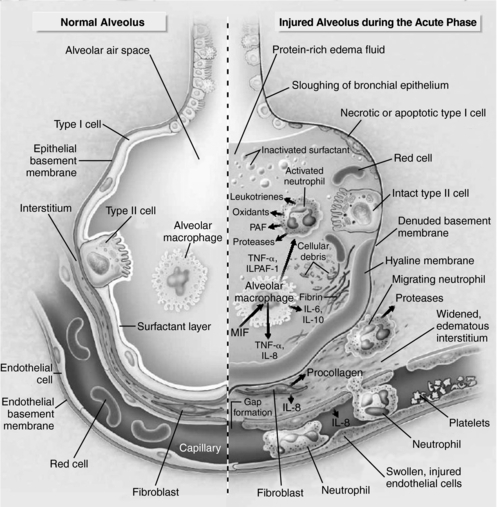 |
| Fig. 2.1A normal alveolus (left) and an injured alveolus (right) in the acute phase of lung injury and acute respiratory distress syndrome.(Modified from Ware, L. B., & Matthay, M. A. [2000]. Acute respiratory distress syndrome. New England Journal of Medicine, 342(18):1334-1349.) |
The exudative phase of ARDS is characterized by increased permeability of the alveolar-capillary barrier. The increased permeability results when type I pneumocytes are damaged and slough off, exposing the basement membrane. This allows the influx of protein-rich edema fluid into the airspaces and accumulation of fluid along the alveolar wall, forming a hyaline membrane. As a result, lung volume and pressures increase, causing pulmonary edema and subsequently leading to changes in hydrostatic pressure and, ultimately, impaired diffusion of oxygen (Weinacker & Vaszar, 2001; Tomashefski, 2000). In addition, type II pneumocytes become hyperplastic (Weinacker & Vaszar, 2001). The injury to type II cells results in impaired fluid transport, removal of edema fluid from the alveolar space, and a decrease in surfactant production and turnover (Tomashefski, 2000; Ware & Matthay, 2000).
During the exudative phase of ARDS, neutrophils are recruited to the interstitium in response to cellular adhesion molecules (e.g., selectins and beta-2 integrins) and subsequently are activated by a number of cytokines (e.g., tumor necrosis factor and interleukin-1 [IL-1], IL-6, IL-8, and IL-10) and complement (enzymatic serum proteins), which are thought to induce and sustain an inflammatory response (Weinacker & Vaszar, 2001). Although neutrophils are part of the inflammatory process, they do not play an essential role in overall lung injury, as evidenced by the fact that neutropenic patients can develop ARDS (Mokart et al., 2003). Moreover, the lungs have a reservoir of alveolar and interstitial macrophages. Macrophages not only play an important roll in proinflammatory effects in the lungs, they also have a potentially crucial roll in the antiinflammatory effects and in the resolution of acute lung injury (Pittet et al., 1997).
Furthermore, anticoagulant proteins (protein C and protein S) decrease, and procoagulant proteins (tissue factor) and antifibrinolytic proteins (plasminogen activator inhibitor-1 [PAI-1]) increase. This leads to platelet aggregation and, ultimately, increasing pulmonary vascular resistance. It also contributes to pulmonary hypertension (Bernard, 2005; Weinacker & Vaszar, 2001).
Fibroproliferative Phase
If the exudative phase is sustained, fibroproliferation occurs. This is characterized by the infiltration of fibroblasts and the persistence of inflammation within the interstitium (Weinacker & Vaszar, 2001; Tomashefski, 2000). The type II pneumocytes continue to proliferate and accumulate along the basement membrane, ultimately replacing the type I pneumocytes (Tomashefski, 2000). During this stage, hyaline membranes are no longer formed; instead, the process is complicated by the deposition of collagen by fibroblasts, resulting in thickening of the alveolar walls and, eventually, inhibition of lung compliance (Weinacker & Vaszar, 2001).
Fibrotic Phase
The final stage of ARDS is the fibrotic phase, which is manifested by fibrosis, scarring, and cyst formation (Weinacker & Vaszar, 2001). The lung becomes completely remodeled by sparsely cellular collagenous tissue (Tomashefski, 2000).
EPIDEMIOLOGY AND ETIOLOGY
For decades ARDS went by a plethora of names (e.g., DaNang lung, shock lung, post-traumatic lung) (Bernard et al., 1994). The condition first was described as “acute respiratory distress syndrome” in 1967, in a historic article by Ashbaugh and colleagues in the journal Lancet (Ashbaugh et al., 1967). Subsequently, ARDS was frequently referred to as “adult respiratory distress syndrome” to distinguish it from infant respiratory distress syndrome (Ashbaugh et al., 1967). In 1992, the American-European Consensus Committee on ARDS (AECC) was designated to standardize a definition of ARDS and to address the many therapeutic options, as well as prevention of the syndrome in individuals at increased risk (Bernard et al., 1994). This had a positive impact on clinical and epidemiologic research, one that continues to benefit patient survival and overall health-related quality of life (HRQOL) (Heyard et al., 2005; Bernard et al., 1994).
The AECC determined that acute rather than adult best describes the syndrome because the condition is not strictly limited to adults (Bernard et al., 1994). The AECC also instituted a new term, acute lung injury (ALI), to encompass a broader range of pathologic processes; ARDS was reserved for the severest end of the spectrum (Bernard et al., 1994). Therefore all patients with ARDS have ALI, but not all patients with ALI have ARDS (Bernard et al., 1994). The criterion for distinguishing between ALI and ARDS is the degree of hypoxemia (Bernard et al., 1994). ALI is defined as a PaO2/FiO2 of less than 300 mm Hg; ARDS is defined as a PaO2/FiO2 of less than 200 mm Hg (Bernard et al., 1994).
In 1972 the National Institutes of Health (NIH) reported the incidence of ARDS as 75 cases per 100,000 population per year in the United States (Bernard et al., 1994). Subsequent studies have suggested that this may be an overestimation caused by the heterogeneity of underlying disease processes and the lack of a standardized definition. Current estimates range from 1.5 to 8.3 cases per 100,000 (Bernard et al., 1994). Recent prospective studies show promise for accurately gauging the incidence of ARDS because these studies use the criteria established by the AECC, which coincidently reflect the original NIH estimates of the incidence of ARDS (Rubenfeld et al., 2005; Goss et al., 2003). The prevalence of ARDS by procedure is reported as 7.9% for pneumonectomy, 2.96% for lobectomy, and 0.88% for sublobar resection (Dulu et al, 2006).
Since the introduction of ARDS into the medical literature, patient mortality has been estimated to exceed 50% (Ware & Matthay, 2000; Milberg et al., 1995; Suchyta et al., 1997). This is similar to the mortality rate for trauma patients in general (Salim et al., 2006). Advances in supportive care (e.g., changes in the method of mechanical ventilation, early and aggressive use of antibiotics, stress ulcer prophylaxis, and improved nutritional and fluid therapy), as well as early identification of risk factors, have resulted in an overall decrease in the mortality of patients who develop ARDS to about 30% to 36% (Jardin et al., 1999; Milberg et al., 1995). This statistic applies to patients 60 years of age or younger who developed ARDS as a secondary complication of trauma or a noninfectious condition. In this patient group, the overall morality rate for those in the intensive care unit (ICU) dropped from 38% to 30%, and the mortality rate for ARDS patients in the hospital overall dropped from 52% to 37% (Ely et al., 2002; Ware & Matthay, 2000; Milberg et al., 1995). In many studies, local immunosuppression appears to be associated with a good prognosis; however, this does not necessarily apply to neutropenic patients. In fact, several studies suggest an increased mortality in neutropenic patients (57% to 80%) compared with those who are not neutropenic (45%) (Mokart et al., 2003)
RISK PROFILE
The ability to identify patients at risk of developing ARDS is essential to preventing the syndrome and to treating patients earlier, thereby reducing the likelihood of severe complications. Multiple studies have shown that sepsis, whether of pulmonary or nonpulmonary causes, is the leading risk factor for the development of ARDS (18% to 43% of cases) (Stapleton et al., 2005; Fein & Calalang-Colucci, 2000; Hudson et al., 1995). The risk increases if sepsis is coupled with systemic hemodynamic responses, impaired perfusion, or multiple organ failure (Ely et al., 2005; Fein & Calalang-Colucci, 2000; Gattinoni et al., 1994). Research also shows that if sepsis is complicated by prolonged hypotension, disseminated intravascular coagulation (DIC), or shock, the incidence of ARDS increases (Fein & Calalang-Colucci, 2000).
Other factors that have been shown to increase the risk of ARDS include age over 68 years; female gender; severe illness (including chronic obstructive pulmonary disease [COPD], asthma, and bronchitis); malignancies (including malignant lymphoma and acute promyelocytic leukemia) (Kamikura et al, 2006; Larson & Tallman, 2003); opportunistic infections (including adenovirus and vancomycin-resistant enterococcus) (Wallot et al., 2006; Avery et al., 2005); cigarette smoking; chronic alcohol abuse; use of amiodarone (Charles et al., 2006); surgery (including lung resection and pelvic exenteration) (Dulu et al., 2006; Wydra et al., 2006); and treatment-related pulmonary toxicities (e.g., chemotherapeutic agents and radiation) (Hudson et al., 1995; Iribarren et al., 2000).
Patients with oncologic malignancies undergoing treatment with chemotherapy are at an even greater risk of developing ARDS. The pathogenesis is thought to be caused by hemostatic abnormalities, leukocyte activation, and possibly deactivation of alveolar macrophages (Kamikura et al., 2006; Mokart et al., 2003).
PROGNOSIS
Researchers have elucidated a significant proportion of the underlying pathogenicity of ARDS in the hope of finding specific biomarkers that would enhance diagnosis and prognosis and help identify patients at the highest risk of developing the condition (Pittet et al., 1997). As yet, no consistent biomarker has been identified that can be applied to clinical practice.
Nevertheless, our increased knowledge of this complex syndrome allows us to better understand the causes of increased mortality in ARDS patients. Several studies have consistently shown that sepsis coupled with multiple organ failure is the most common cause of death (30% to 50% of cases) (Stapleton et al., 2005; Milberg et al., 1995; Hudson et al., 1995). A correlation exists between sepsis that persists 72 hours or longer after the onset of ARDS and patient survival (Fein & Calalang-Colucci, 2000). In addition, the degree of alveolar-epithelial injury, as well as structural changes, is associated with a higher patient mortality and can be a reliable predictor of overall outcome and HRQOL (Heyard et al., 2005; Ely et al., 2002; Gattinoni et al., 1994).
Patients over age 70 who develop ARDS have a higher mortality rate than patients under age 70 who develop the syndrome, even when gender, multiple organ failure, or sepsis is taken into account. This situation is attributed primarily to the duration of ventilator support. It also may reflect age bias, which can influence the decision on whether to withdraw support or to initiate end-of-life discussions with the families of patients who are likely die (Stapleton et al., 2005; Ely et al., 2002; Suchyta et al., 1997).
Advances also have been made in the treatment options for patients who survive ARDS, prompting clinical research directed at evaluating survivors of ARDS and their HRQOL. These studies have uniformly shown an overall improvement in aspects of physical function at 6 months and 12 months, although with some physical limitations (Heyard et al., 2005; Herritage et al., 2003).
Research suggests that a correlation exists between the degree of pulmonary injury, pre-existing co-morbidities, the duration of the critical illness, and possible treatment-related side effects and the patient’s HRQOL (Heyard et al., 2005; Herritage et al., 2003).
PROFESSIONAL ASSESSMENT CRITERIA (PAC)
Clinically ARDS usually occurs in the setting of critical illness. Frequently it reflects the underlying pathology that can co-exist, and it may obscure and possibly delay diagnosis. The first, or acute, phase occurs at the time of injury; symptoms usually develop within 12 to 48 hours of the inciting event (Steinberg & Hudson, 2000). Physical examination findings in this phase most often are minimal. The condition is manifested initially by exertional dyspnea that results in arterial hypoxemia refractory to supplemental oxygen (a classic feature) (Ware & Matthay, 2000). At this point, the chest x-ray findings are not usually diagnostic and can be indistinguishable from those of cardiogenic pulmonary edema (Ware & Matthay, 2000).
Within several hours to days, the latent phase ensues. Bilateral, patchy, ill-defined radiographic infiltrates gradually develop, and fine rales may be auscultated during the physical examination (Steinberg & Hudson, 2000). The latent phase is followed by acute respiratory failure. The patient becomes increasingly dyspneic, tachypneic, and hypoxemic as a result of decreased lung compliance, and diffuse rales can be auscultated. Chest x-ray films show the progression of patchy, coalescing infiltrations (Weinacker & Vaszar, 2001).
The final phase of ARDS is manifested by intrapulmonary shunting that leads to refractory hypoxemia and metabolic and respiratory acidosis. The chest x-ray films show a more reticular pattern, which represents the beginning of fibrosis (Weinacker & Vaszar, 2001).
1. Vital signs: Tachypnea, tachycardia, hypertension, chest discomfort, fever
2. History: See risk profile.
3. Hallmark physical signs and symptoms: Dyspnea
4. Additional signs and symptoms: Evidence of respiratory muscle fatigue: intercostal retractions, paradoxical chest and abdominal movement; faint or pronounced rales or cough
5. Psychosocial signs: Anxiety
6. Laboratory values and diagnostic tests: Early evidence of varying degrees of hypoxemia (generally resistant to oxygen supplementation); respiratory alkalosis, decreased PCO2; late evidence of arterial metabolic or respiratory acidosis; widened alveolar-arterial gradient; ANC less than 500 neutrophils/mm3; PaO2/FiO2 less than 300 mm Hg for ALI; less than 200 mm Hg for ARDS (Bernard et al., 1994); CXR: initially may be normal, with progression to bilateral pulmonary infiltrates; blood and urine cultures; fluid from bronchoalveolar lavage (reserved for selected patients) shows thioredoxin level greater than 61 ng/mL (Callister et al., 2006).
NURSING CARE AND TREATMENT
Supportive therapy is the basis of treatment for ARDS. It is directed toward identifying and managing pulmonary and nonpulmonary organ dysfunction (Brower et al., 2001). All inciting etiologies should be excluded. In addition, because of the increased mortality related to sepsis, a high index of suspicion should be maintained for potentially treatable infections, and prompt antimicrobial intervention or surgery should be provided as necessary (Ware & Matthay, 2000).
Essential Nursing Care
• Evaluation of chest x-ray films
• Computed tomography (CT) scan evaluation
• Mechanical ventilation
• Blood, urine, and sputum culture evaluations
• Nutritional support
• Intravenous antibiotic intervention
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
