- Function of the five different kinds of white cell
- Defining a total and differential white cell count
- Defining some white cell related terms
- Causes and consequences of increased white cell count
- Causes and consequences of decreased white cell count
- A brief introduction to leukaemia – a malignant disease of white cells
In this second of two chapters devoted to the most commonly requested blood test – the full blood count (FBC) – we consider one element of it: the white blood cell count (WBC). Unlike the mature red cell population, which is homogenous in nature, the white cell (leucocyte) population is heterogeneous, composed as it is of five morphologically and functionally distinct populations. These are: neutrophils, eosinophils, basophils, monocytes and lymphocytes. The total WBC is the sum of all of these white cell types, whilst the differential WBC or ‘diff’ is a count of each of the five types. An increase in white cell numbers is a very common feature of disease, which can be attributed to one of several pathological processes, including the big three: infection, inflammation and malignancy. A reduced white cell count, which is much less common, implies a reduction in immunity and therefore high risk of infectious disease.
Normal physiology
In common with all other formed elements in blood, white cells (leucocytes) are derived from the pluripotent stem cell present in bone marrow (see Chapter 15, Figure 15.1). Mature white cells have a limited life span so that constant bone marrow production is necessary throughout life. An increase in bone marrow production of white cells is part of the body’s normal (inflammatory) response to any insult to the body (i.e. any tissue injury, whatever the cause). The purpose of the inflammatory response is to contain and control injury, to eliminate potential pathogens (bacteria, viruses, fungi, protozoa, parasitic worms) and initiate healing and tissue repair. As key players in the inflammatory response white cells must leave the blood and enter the tissues. Although, as we shall see, each type of white cell has a different and well-defined job to do in the overall process of inflammation, they operate in concert, communicating via a range of chemical messengers called cytokines.
Neutrophils
Comprising 40–70% of the total white cell population, the neutrophil is the most abundant of all white cells in blood. The mature neutrophil has a multi-lobed purple staining nucleus and dark blue staining granules in the cytoplasm. It has a diameter of about 15 μm, around twice that of a red cell. The function of these cells is to enter the tissues and kill invading bacteria. On release from the bone marrow, mature neutrophils spend only around eight hours in the blood stream and the rest of their four to five day maximum life span in the tissues. Chemicals called chemotactic factors released from bacteria and other cells (including basophils, macrophages and lymphocytes, explained further) attract neutropils to the site of tissue infection or inflammation. In the tissues, neutrophils surround and engulf bacteria by a process called phagocytosis (destruction). Once inside the neutrophil, bacteria are killed by the action of enzymes and highly reactive free radical chemicals produced within the darkly staining granules of the neutrophil cytoplasm. Pus, the yellowish fluid that oozes from some bacterially infected sites, is a visible reminder of neutrophil function. It is largely composed of dead and dying neutrophils, bacterial debris and other cellular detritus produced during the fight against infection caused by pyogenic bacteria.
Eosinophils
Although much fewer in number, comprising only 0.2–5% of total white cell population, eosinophils have similarities, both in appearance and function, to neutrophils. The nucleus of the eosinophil is like the neutrophil, lobed in appearance, though whereas the neutrophil nucleus is multi-lobed, the eosinophil nucleus has just two lobes. The granules of the eosinophil cytoplasm stain orange-red in contrast to the dark blue of the neutrophil, due to the presence of chemicals peculiar to the eosinophil. Like neutrophils, eosinophils are capable of phagocytosis, although it seems unlikely that they have a role in the killing of bacteria. Instead they target foreign material too large for normal neutrophil mediated phagocytosis. For example, they bind to parasitic worms and inflict damage by releasing enzymes, and then phagocytose the products of this enzymic destruction. Their main function then is protection against infection by organisms larger than bacteria and viruses. Eosinophils are recruited to the site of inflammation caused by allergic reactions, such as the airways of allergic asthma and hay fever sufferers. Chemicals released from ‘activated’ eosinophils at these sites contribute to the pathogenesis of allergic inflammatory disease.
Basophils
These are so few in number that they are only rarely seen during microscopical examination of blood. They have a multi-lobed nucleus that is hidden by dense dark blue staining granules. Basophils migrate into tissues where they mature to mast cells. When activated, mast cells release many chemical mediators of the inflammatory response, which include a chemotactic factor that attracts neutrophils, histamine a chemical which dilates blood vessels, increasing the blood flow to damaged areas, and heparin, an anticoagulant required to begin the process of repair to damaged blood vessels.
Monocytes
The monocyte is the largest blood cell and has a lobulated nucleus, with a usually clear (non-granulated) voluminous cytoplasm. After a short period of 20–40 hours circulating in the blood, these phagocytic cells migrate to the tissues where they mature to cells called macrophages. Macrophages phagocytose and kill foreign organisms in the same way that neutrophils do but have a second important role in processing and presenting foreign proteins or antigens (derived from bacteria etc.) to T-lymphocytes for initiation of a cell mediated immune response (discussed further). Macrophages also have an important physiological role in the controlled destruction of red cells when they are no longer viable. This is discussed in Chapter 15.
Lymphocytes
Between 20% and 40% of the circulating white cell population are lymphocytes; these are the second most abundant type of white cell in blood. Like all other blood cells they are derived from the bone marrow, but a proportion undergo further processing within the thymus; these are thymus dependent lymphocytes or T-lymphocytes, which comprise around 70% of all circulating lymphocytes. Most of the remaining 30% are B-lymphocytes. There is an additional small population of non-B, non T-lymphocytes called natural killer (NK) lymphocytes. The routine lymphocyte count is the sum of these three types.
Like all other white cells, lymphocytes are required for immunity (protection) from infection. B-lymphocytes differentiate to plasma cells, which produce antibodies. These are proteins that bind specifically with complementary proteins called antigens. Micro-organisms (bacteria, viruses etc.) all have specific surface proteins which act as antigens. Antibody binding of these surface antigens prevents bacteria and viruses from invading tissue cells. Furthermore antibody coated bacteria are much more easily phagocytosed (destroyed) by neutrophils and macrophages. Antibodies can also bind to, and thereby neutralise, bacterial toxins.
Although antibodies are effective in the process that leads to destruction of microbes outside cells, they cannot enter cells and therefore have no effect on the microbes harbouring within cells. The body’s defence against these depends largely on NK- lymphocytes and T-lymphocytes.
T-lymphocytes can ‘recognise’ and destroy tissue cells that are infected, thereby preventing further spread. Since all viruses must infect cells in order to replicate, and many bacteria parasitise body cells, this so called cell mediated immunity invoked by the T-lymphocyte is a vital component of the body’s defence against infection. T-lymphocytes (and NK-lymphocytes) also have the capacity to recognise and kill cancerous cells, so are part of the body’s defence against cancer.
An important feature of lymphocyte mediated immunity, whether it be B-lymphocyte (i.e. antibody) mediated or T-lymphocyte (i.e. cell) mediated is that, unlike all other white blood cells, both classes of lymphocytes have the capacity to ‘remember’ an invading organism, so that the response to a subsequent encounter is greater and more rapid. This so called ‘acquired’ immunity explains why we seldom suffer more than once from a particular infection. First exposure provides increased immunity from subsequent infections with the same organism. The function of NK-lymphocytes is not dependant on this ‘acquired’ immunity mechanism, but in common with non-lymphocyte white blood cells (neutrophils, eosinophils, basophils and monocytes), they are part of what is known as the body’s ‘innate’ immunity, that is the immunity we are born with.
Laboratory measurement of full blood count
Interpretation of WBC and diff results
Approximate reference ranges
Total white blood cell (WBC) count:
Adult males | 3.7–9.5 × 109/L |
Adult females | 3.9–11.1 × 109/L |
Differential white cell count:
Neutrophils (40–75% of total white cells) | 2.5–7.5 × 109/L |
Lymphocytes (20–40% of total white cells) | 1.5–4.0 × 109/L |
Monocytes (2–10% of total white cells) | 0.2–0.8 × 109/L |
Eosinophils (1– 5 % of total white cells) | 0.04–0.44 × 109/L |
Basophils ( <1% of total white cells) | 0.01–0.10 × 109/L |
At birth the total white cell count is very high (9–26 × 109/L). This falls sharply to around 5–18 × 109/L during the first two months of life and to normal adult levels by 12–15 years of age.
Critical values
White blood cell count (WCC) < 2 × 109/L or > 30 × 109/L.
Terms used in interpreting results
Polymorphonuclear cells (polymorphs) | literally ’many shaped nucleus’ cells, refers to all whiteblood cells with a lobed nucleus ie neutrophils, eosoinophils, and basophils. Lymphocytes and Monocytes are non-polymorphs because they have a more regular shaped nucleus. |
Granulocytes | all white cells with visibly staining granules in the cytoplasm (i.e. neutrophils, eosinophils and basophils.) Lymphocytes and monocytes are non-granulocytes. |
Agranulocytosis | complete or near absence of granulocytes in blood. |
Phagocytes and | phagocytes are cells that are able to phagocytose |
non-phagocytes | foreign material (bacteria etc.). Neutrophils, eosinophils, basophils and monocytes are all phagocytes. Lymphocytes do not have this ability; they are non-phagocytes. |
Leucocytosis | an increase in total white cell count. |
Neutrophilia, eosinophilia, | a selective increase in neutrophil, eosinophil and |
basophilia | basophil count respectively. |
Lymphocytosis | an increase in lymphocyte count. |
Leucopaenia | a reduced total white cell count. |
Neutropaenia | a reduced neutrophil count. |
Lymphopaenia | a reduced lymphocyte count. |
Pancytopaenia | a reduction in all blood cells (red cells, white cells and platelets). |
Terms used to describe white cells when viewed under the microscope
Increase in band forms | band cells are slightly immature neutrophils recognisable by the non-segmented shape of the nucleus. Normally only 3% of total neutrophils in peripheral blood are of this sort. An increase implies the bone marrow is increasing white cell production in response to infection or inflammation. |
Shift to left | an alternative term to ‘increase in band cells’ denoting increase in immature neutrophils in peripheral blood. |
Blast cells | primitive white cells never normally seen in peripheral blood. Their presence almost always indicates an acute haematological malignancy (e.g. acute leukaemia). |
Causes of an increase in total white cell numbers
General considerations
An increase in white cell numbers (leukocytosis) occurs most commonly as a result of infection, inflammation or indeed any significant tissue damage. Since the role of white cells is to defend the body against infection it is entirely appropriate that numbers should increase under these circumstances. This so called benign or reactive leukocytosis must be distinguished from the much less common and entirely inappropriate leukocytosis that may be a feature of haematological malignancies, including the leukaemias.
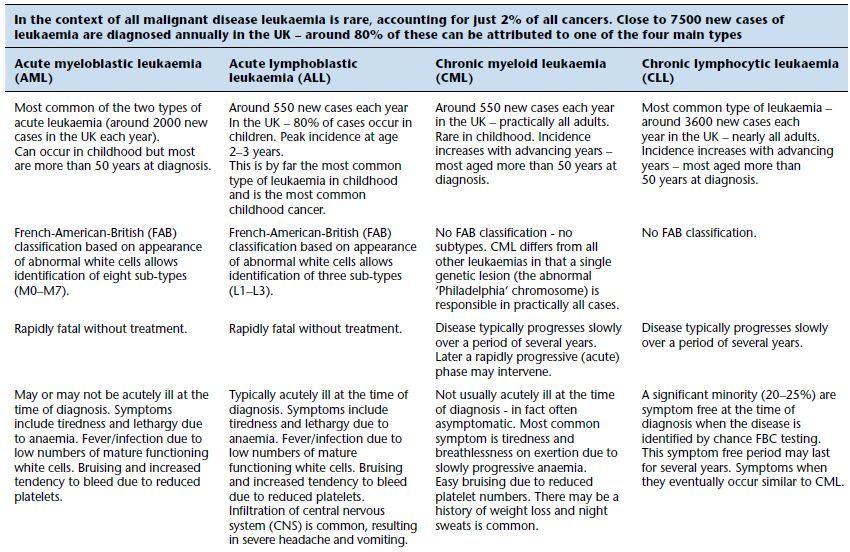
The leukaemias are a group of malignant diseases of the bone marrow, characterised by the unregulated proliferation of one sort (a clone) of immature white blood cell at the expense of normal blood cell production. Nearly all cases can be classified to one of four groups depending on whether the clinical course of the disease is rapid (acute) or slow (chronic) and whether the immature cancerous cells are derived from the myeloid bone marrow cells (which normally mature to neutrophils, eosinophils basophils or monocytes) or lymphoid bone marrow cells (which normally mature to lymphocytes). The four main types of leukaemia then are: acute myeloid leukaemia (AML), chronic myeloid leukaemia (CML), acute lymphoblastic leukaemia (ALL) and chronic lymphoid leukaemia (CLL). Some of the distinguishing features of the four sorts of leukaemia are outlined in Table 16.1. Since in all cases normal blood cell development is impaired, anaemia (due to deficiency of normal red cells), impaired blood coagulation with increased tendency to bleed (due to low platelet count) and high risk of infection (due to reduced numbers of normal white cells) can be features of all leukaemias.
Whether it be benign (reactive) or malignant, leukocytosis is usually the result of a predominant, although not necessarily entirely selective, increase in one of the five types of white cell. The differential count thus provides clues as to the cause of an increased total white cell count. A more detailed account of the cause of increased white cell numbers will be given focusing on each type of white cell in turn.
Causes of increased neutrophil count (neutrophilia)
Neutrophilia is the most common derangement of white cell numbers.
Reactive neutrophilia is a feature of:
- Most acute bacterial infections. Particularly high (up to 50 × 109/L) in pyogenic (pus forming) infections, e.g. those caused by Staph and Strep bacterial species.
- Non-infective acute inflammation (e.g. rheumatoid arthritis, inflammatory bowel disease etc.).
- Tissue damage (surgery, trauma, burns, myocardial infarction).
- Solid tumours, e.g. lung cancer (an appropriate response to the tissue necrosis (death) which accompanies tumour growth).
- Extreme physical exercise.
- Pregnancy and labour of pregnancy.
Table 16.1 Some features of the four main types of leukaemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


