The human body uses a complex interaction of systems to maintain homeostasis. As a component of homeostasis, the body maintains a temperature between 36.1° C (97° F) and 37.4° C (99.3° F), with cyclic fluctuations throughout the day. Variations above or below these “normal” parameters can be a signal of disease. For the patient with cancer, fever may be defined as one temperature reading greater than 38.3° C (101° F) or three readings, 1 hour or more apart, greater than 38° C (100.4 °F) within 24 hours (Dalal & Zhukovsky, 2006). It is imperative to recognize that for the patient with cancer, especially the patient with neutropenia, fever may be the initial and only indicator of a change in status and a need for immediate intervention.
Elevation of the core body temperature has long been recognized as a symptom of the onset of disease. However, an elevated temperature is a single component of a larger and more complex physiologic response to disease called the febrile response (Fig. 16.1). During the febrile response, a complex interaction of immunologic, endocrine, behavioral, neural, and cytokine-mediated processes occurs to alter the body’s temperature (Mackowiak, 2006). These systems are coordinated by the hypothalamus to maintain a homeostatic core body temperature.
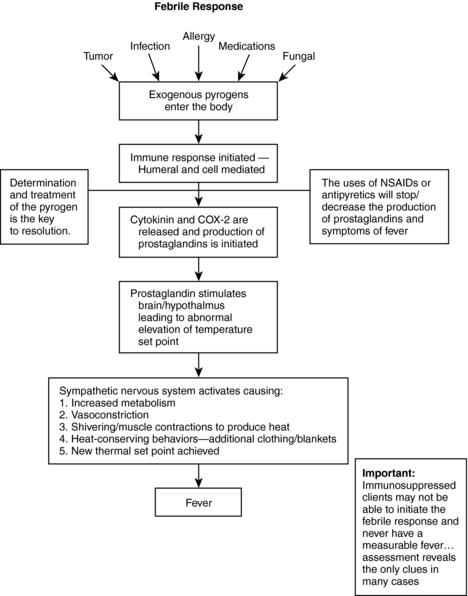 |
| Fig. 16.1The febrile response. |
The hypothalamus is considered the thermostat for the body, and it is an integral component of the febrile response. The body normally maintains temperature through the balanced production and dissipation of heat. However, external factors, such as an increased external temperature or the introduction of endogenous or exogenous pyrogens, can cause regulatory malfunction in the hypothalamus, resulting in a hyperthermic state. During this hyperthermic state, or fever, a new hypothalamic set point is initiated for the body’s core temperature.
Fever has three routinely accepted phases: chill, fever, and flush. During the initial chill phase, the sympathetic nervous system is activated and vasoconstriction occurs in the cutaneous blood vessels. Shivering may begin to generate heat through muscle activation. Sweat production is inhibited and neurotransmitters are secreted, leading to increases in cell metabolism, heat production, and body temperature (Dalal & Zhukovsky, 2006). During this initial phase, the individual may perceive himself or herself to be cold and may try to get warm, perhaps by using blankets or putting on extra clothing. These behavioral actions contribute to heat conservation and raise the body’s core temperature to the new set point. However, in a patient with cancer, the immune system may be blunted to such an extent that the person is unable to mount even the first sign of infection or a mild febrile response. In addition, patients with cancer are subject to polypharmacy; antipyretics may be given before a blood transfusion, and corticosteroids may be used to reduce inflammation. All these factors may diminish the body’s ability to recognize a pyrogen and release the appropriate mediators to initiate a febrile response.
Once the new hyperthermic set point is reached, heat production is balanced by heat loss, and the body maintains this temperature through negative feedback mechanisms. Heat-sensitive receptors in the hypothalamus and throughout the body recognize the elevated temperature and reduce the stimulation of the sympathetic nervous system, thereby maintaining the newly elevated body temperature. This is the second, or fever, phase.
In the final phase of fever, the flush phase, the hypothalamus recognizes resolution of the cause of the elevated temperature and returns to the routine body temperature. Once the pyrogens and toxins have been controlled or antipyretic therapy has been initiated, the hypothalamus initiates a reverse cycle in which the sympathetic nervous system is inhibited. This causes cutaneous vasodilation, diaphoresis, and a decrease in cell metabolism. These mechanisms promote heat loss and the body’s return to the new, lower set point.
Cytokine Mediators
When exogenous pyrogens enter a host, an inflammatory response is initiated. Macrophages and phagocytic cells recognize an invader, and proinflammatory cytokinines are produced and released into the circulation, stimulating the production of prostaglandins. The cytokinines include interleukins 1B (IL-1B), 6 (IL-6), interferon, and tumor necrosis factor (TNF). In addition to producing prostaglandins, these cytokinin mediators are thought to stimulate central production of the inducible enzyme cyclooxygenase-2 (COX-2) (Dalal & Zhukovsky, 2006). Current research suggests that it is this prostaglandin production that activates the thermoregulatory aspects of the hypothalamus and begins the febrile response and fever. Another factor is that a person loses about 1% of the body’s immune function each year after age 30; therefore a person who is 80 years old has about 50% of his or her original immunity, which leads to lower regulation levels.
EPIDEMIOLOGY AND ETIOLOGY
Patients with cancer are a subgroup of patients who may be both immunocompromised and physically debilitated (Smeltzer & Barel, 2004). Consequently, recognizing and diagnosing the cause of fever in a patient with cancer often is a difficult and time-consuming endeavor. In these patients, the signs and symptoms that signal a change in their condition may be subtle and difficult to differentiate. It is imperative that clinicians be alert for and recognize subtle signs and symptoms and that they evaluate each as the potential initiator of fever.
Typically, fever in the oncology patient is caused by infection, the tumor itself (paraneoplastic fever), medications, blood product transfusions, and graft versus host disease. Other, less frequent causes include narcotic drug withdrawal (specifically benzodiazepines), neuroleptic malignant syndrome, and obstruction of a viscus organ (e.g., bowel or bladder). In addition, co-morbid conditions should be evaluated as a cause of fever. Cerebrovascular accidents, thrombosis, and connective tissue disorders may also induce a hyperthermic state.
Infection
Infection is one of the most common causes of fever in patients with cancer, and it is associated with high rates of morbidity and mortality. Without rapid intervention and treatment (i.e., within 48 hours), the mortality rate among patients with cancer who have a fever caused by infection can be as high as 70%. The severity of infection is inversely proportional to the number of circulating neutrophils; therefore patients who are neutropenic are at a much higher risk for more frequent and severe infections than patients who are not immunocompromised. In patients with neutropenia (defined by an absolute neutrophil count [ANC] of less than 500 cells/mm3), fever should be considered an absolute emergency and intervention begun immediately. An additional factor in predicting outcomes is the nadir of the neutropenic causative agent and the nadir of the neutropenia itself. An antineoplastic agent with a short nadir leads to rapid depletion of neutrophils and puts the patient at much higher risk of infection, sepsis, and death. One area that requires continued research in oncology is the relationship between chemotherapeutic agent nadirs and patients’ risk for neutropenia. Certain chemotherapeutic regimens (Crawford et al., 2005) correlate with a higher risk of neutropenia (Table 16-1). Research seems to support the prophylactic use of colony-stimulating factors in these high-risk patients (Rolston, 2004).
| Type of Cancer | Treatment |
|---|---|
| Breast cancer | Doxorubicin, cyclophosphamide, docetaxel (ACT) Doxorubicin, paclitaxel (AT) Docetaxel, doxorubicin, cyclophosphamide (TAC) |
| Non-Hodgkin’s lymphoma | Vincristine, doxorubicin, prednisone, etoposide, bleomycin, cyclophosphamide (VAPEC-B) Dexamethasone, cisplatin, cytarabine (DHAP) Etoposide, methylprednisolone, cisplatin, cytarabine (ESHAP) |
| Ovarian cancer | Topotecan Paclitaxel Docetaxel |
| Testicular cancer | Vinblastine, ifosfamide, cisplatin (VIP) |
| Non-small cell lung cancer | Cyclophosphamide, doxorubicin, etoposide (CAE) Topotecan Topotecan, paclitaxel (Top T) |
Neutropenic patients are at the highest risk for infection (Baden & Rubin, 2002). The organisms that cause the infections are related to the geographic and site-specific origin of the infection. The most common sites of infection in patients with cancer are the pharynx, lower esophagus, lung, perineum, anus, eye, skin, periodontium, vascular catheter access sites, and tissue around the nails (Urabe, 2004). Some common organisms have been associated with specific sites (Table 16-2) (Hughes et al., 2002). The clinician should pay close attention to these sites and inspect and evaluate any redness, tenderness, drainage, or abnormal conditions. As mentioned, in oncology patients the body’s ability to mount an effective immune response may be significantly blunted, and subtle abnormalities may be the only signal of infection.
| Site | Cause | Symptoms |
|---|---|---|
| Cutaneous | S. aureus, streptococci | Cellulitis, abscesses, folliculitis |
| Pulmonary | S. aureus, P. aeruginosa | Adventitious breath sounds, cough |
| H. influenzae, S. pneumoniae | fever, dyspnea | |
| Sepsis | S. aureus, P. aeruginosa, | Hypotension, tachycardia, |
| Enterobacter spp. | decreased mentation, | |
| nausea/vomiting | ||
| UTI | E. coli | Urgency and frequency in voiding |
| Insertion sites/catheters | S. aureus/coagulase negative | Redness at site and sepsis symptoms |
| Staphylococci, P. aeruginosa |
A significant proportion of the fever-causing infections in patients with cancer now are gram positive in nature. The main culprits are staphylococci (S. aureus and S. epidermidis), streptococci (e.g., S. pneumoniae, a common cause of pneumococcal pneumonia), enterococci, and strep A and B. These organisms must be treated; however, immediate initiation of a glycopeptide antimicrobial (vancomycin) is not necessarily required unless the patient’s symptoms are consistent with sepsis or an exact pathogen has been identified.
Gram-negative organisms are a less likely pathogen in patients with cancer. However, gram-negative bacilli and cocci are potentially the most deadly and therefore require immediate treatment to prevent sepsis and progression. Because gram-negative organisms can be isolated from multiple sites in a patient with cancer, the sites of origin (e.g., urinary tract, pulmonary and gastrointestinal tracts) must be evaluated extensively for potential infection-causing organisms. Gram-negative organisms (e.g., Escherichia coli and Klebsiella and Pseudomonas spp.) require immediate, broad-spectrum antimicrobial coverage because of the potentially rapid and overwhelming effects of sepsis in a patient with cancer. Even though gram-positive organisms are seen more often in these patients, progression of gram-negative infections is associated with a higher mortality rate (Klastersky, 2004).
Fungal Infections
During the initial neutropenic episode, bacterial infections are most prevalent. However, as the host remains neutropenic, the risk for fungal infections increases. Fungal infections in a patient with cancer can be either a primary cause of illness or a secondary opportunistic infection that occurs in a patient being treated with antimicrobials. By far the most common cause of fungal infections is Candida albicans, and Aspergillus organisms are a distant second (Wingard, 2004). In a patient with fever, especially a neutropenic patient, fungal infection is often seen when the patient is not responding to broad-spectrum antimicrobial therapy. The clinical manifestations of both Candida and Aspergillus infections have a high likelihood of fever associated with infection (Wingard, 2004) and may mimic symptoms of a bacterial infection.
A continuing challenge for health care providers is how to rapidly identify patients experiencing fever who are potentially neutropenic and in need of antifungal therapy. In the past, empiric antifungal therapy usually consisted of deoxycholate (amphotericin B [Amp B]). However, this antifungal medication has been associated with serious toxicities, including nephrotoxicity, phlebitis at the infusion site, and anemia. Although amphotericin B is still used, newer antifungals have emerged. Among these are the “azoles” (e.g., fluconazole, itraconazole, ketoconazole, and voriconazole), which have been shown to have fewer toxicities (Wingard, 2004). However, as with any medication regimen, the patient’s response to treatment must be monitored continuously and adjusted as necessary.
Paraneoplastic Fever
Fever is a common associated illness with some cancers and may parallel the progression of the tumor or cancer. Paraneoplastic fevers are most often associated with Hodgkin’s lymphoma. However, several other types of cancer are coming to be associated with paraneoplastic fever. Cancers such as acute leukemia, lymphoma, renal cell carcinoma, bone sarcoma, adrenal carcinoma, and pheochromocytoma are associated with paraneoplastic fever. Cancers involving solid tumors (e.g., breast, lung, and colon cancers) are not normally associated with this type of fever (Dalal & Zhukovsky, 2006). The definitive treatment for paraneoplastic fever is treatment of the underlying malignancy. In the absence of effective antineoplastic therapy, treatment with nonsteroidal antiinflammatory drugs (NSAIDs) is the treatment of choice. A common choice of NSAID for this type of fever is naproxen (Naprosyn), and response to initiation of this therapy has been used to diagnose tumor-associated fevers. However, chronic use of NSAIDs may cause bleeding problems, therefore the patient needs continued follow-up and teaching regarding the signs and symptoms of undesired bleeding, such as black, tarry stools, excessive bruising, and worsening or abnormal fatigue. These symptoms should be reported to the caregiver immediately.
Medication-Related Fever
The diagnosis of medication-induced fever in an oncology patient is often a diagnosis of exclusion, except for specific medications (Box 16-1). These include biologic modifiers (interferon, TNF), bleomycin, and amphotericin B. These drugs routinely cause fevers, and the patient may need to be treated prophylactically before initiation of therapy. Premedication with NSAIDs, acetaminophen, or corticosteroids may lessen the fever. In most cases, a fever related to medication administration resolves once the drug therapy is stopped. Other medications that may cause fever are antibiotics, cardiac medications, antiseizure medications, cytotoxics, and growth factors. In a review study, antibiotic therapy accounted for approximately 31% of drug-related fevers (Dalal & Zhukovsky, 2006). However, patients with cancer are not exempt from cardiovascular disease, diabetes, seizures, and infection, therefore the medications used to treat these conditions should routinely be evaluated in cases of suspected medication-induced fever.
BOX 16-1
MEDICATIONS THAT MAY CAUSE FEVER
5-Flucytosine (Ancobon)
Acycloguanosine (Acyclovir)
Allopurinol (Zyloprim)
Deoxycholate (Amphotericin B)
Bleomycin
Captopril (Capoten)
Cimetidine (Tagamet)
Clofibrate (Clofarabine)
Erythromycin (Ery-tab, Eryc, EES)
Fluconazole (Diflucan)
Heparin sodium
Hydralazine (Apresoline)
Hydrochlorothiazide (HCTZ)
Immunoglobulins (Carimune, Gamunex, Gammagard, BayGam, Flebogamma, RhoGAM, Panglobulin, Polygamy)
Interferons (Avonex, Alferon, Intron, Roferon, Infergen, Rebetron, Rebif, Betaseron, Actimmune)
Isoniazid (INH)
Meperidine (Demerol)
Methotrexate (MTX)
Methyldopa (Aldomet)
Nifedipine (Procardia)
Nitrofurantoin (Marcobid, Macrodantin)
Penicillin’s (Bicillin, Amoxil, Augmentin, Unasyn, Zosyn, Timentin)
Phenytoin (Dilantin)
Procainamide (Procanbid)
Vaccines (Attenuvax, Comvax, Harvix, Meruvax, M-M-R II, Pneumovax, Prevnar, rabies vaccine, Varivax)
Blood Product–Related Fever
Patients with cancer often are treated with antineoplastic medications aimed at killing the rapidly multiplying cancer cells. Because these therapies may also deplete the patient’s blood counts, multiple transfusions of blood products may be required. Fever in response to transfusions is not uncommon in oncology patients. The febrile response is thought to be a result of antibody production by the body as a defense against antigens on the donors’ cells and the resulting inflammatory response. The more transfusions a patient receives, the greater the likelihood of a febrile reaction. Treatment of this type of fever includes premedication with acetaminophen and diphenhydramine. In addition, use of blood products that have been irradiated and depleted of leukocytes can minimize these reactions.
RISK PROFILE
The risk of negative outcomes associated with fever in a patient with cancer directly correlates with the patient’s immune status and exposure to infective agents. As discussed previously, a patient with protracted neutropenia or one who is prescribed an antineoplastic agent with a longer nadir has a greater potential for infection and thus the potential for fever. Patients with cancer who are more at risk for infection and fever can be divided into three categories based on (1) the local epidemiology, with the highest risk seen in hospitalized patients; (2) the type of cancer for which the patient is undergoing treatment, with the highest risk seen in patients with leukemia, lymphoma, brain tumors, or sarcoma; and (3) the patient’s condition at the time of infection, with the highest risk seen in patients who are physically debilitated or at the lowest point in the nadir.
To use the local epidemiology as part of the evaluation and treatment of a patient with fever, the patient’s local environment must be assessed. More precisely, this is the environment in which the patient was exposed to the causative agent or became ill. Such areas include the health care environment and the community environment. The two environments have similar patterns, but their circumstances may dictate different courses of treatment. For example, with nosocomial infections, antibiotics may prove less efficacious, and multiple protocols may be initiated because the organisms may have developed resistance to routine antibiotics. Community-acquired infections may involve more virulent toxins, but they may respond to treatment more quickly. Education of the patient at higher risk for community-acquired infections should center around hygiene (e.g., hand washing), good housekeeping skills, and the need to change furnace and air conditioning filters every month or as specified by the appliance’s manufacturer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


