- Therapeutic value and safety of paracetamol and aspirin
- Mechanisms of toxicity in paracetamol and aspirin overdose
- Signs and symptoms of paracetamol and aspirin overdose
- Plasma paracetamol/salicylate used to assess overdose severity
- Plasma paracetamol/salicylate used to monitor overdose treatment
- Principles of treatment in paracetamol and aspirin overdose
Clinical laboratory staff members are often requested to analyse blood or urine samples for the presence of specific drugs. Such analyses are valuable in two usually separate clinical contexts: deliberate or accidental drug overdose; and therapeutic drug monitoring. Chapter 14 is concerned principally with the latter, whilst this chapter is concerned with how laboratory measurement of serum or plasma concentration of salicylate and paracetamol contributes to the care of patients who have, or are suspected of having, taken a deliberate or accidental acute overdose of aspirin (acetylsalicylic acid) or paracetamol (called acetaminophen in the US). Despite government initiatives to restrict over the counter sales1, paracetamol remains the most common medicinal drug to be taken in overdose, accounting for a half of all drug overdose related attendances at UK hospital emergency departments2. If not promptly treated paracetamol overdose can cause potentially fatal liver damage; it is the most common cause of acute liver failure and necessity for liver transplantation. It has been estimated that paracetamol overdose is the sole or contributory cause of death for around 500 people every year in England and Wales3 and the sole cause of death for 100–150 people. Overdose with aspirin is less common but still accounts for 5–7% of drug overdose related hospital admissions and is solely responsible for around 30–40 deaths in the UK each year4.
Clinical use of aspirin and paracetamol; safety of therapeutic dose
Both aspirin and paracetamol are analgesics and antipyretics, that is they reduce pain and body temperature. They are frequently self-prescribed for the relief of temporary symptoms associated with minor viral infections (e.g. influenza, colds etc.) and for the relief of headaches and minor muscular aches and pains. Aspirin has two further major pharmacological properties: at high dose (>3 g/day) it has anti-inflammatory effect and at lower dose (75–300 mg/day) an anti-thrombotic (anti-blood clotting) effect. These two properties have determined that long-term prescription of aspirin is useful in the treatment of chronic inflammatory conditions like arthritis, and for the prevention of myocardial infarction and strokes among high-risk patients. The efficacy of regular aspirin among the general adult population for primary prevention of myocardial infarction and strokes has been proposed but remains controversial. There is accumulating evidence that long-term aspirin use may protect against many common cancers5; that evidence is now particularly strong and, to all intents and purposes, proven with respect to colorectal (bowel) cancer6,7.
The maximum recommended dose of paracetamol for adults and children over the age of 12 years is two 500 mg tablets, with an interval of at least four hours between doses. No more than eight tablets should be taken in 24 hours (i.e. a maximum dose of 4 g/day). Children aged 6–12 years should be given no more than half the adult maximum single and total 24 hour dose, and children aged 1–5 years no more than a quarter of the adult maximum dose. So long as these dosing recommendations are not exceeded, paracetamol has no adverse effect and is a remarkably safe drug, even if used for a prolonged period.
Long-term aspirin use, even at prescribed therapeutic doses, is less safe. In common with other non-steroidal anti-inflammatory drugs (NSAIDs), long-term aspirin use is associated with irritation of the stomach wall lining (gastric mucosa) and the risk of gastrointestinal bleeding and stomach ulcers. Patients with a history of peptic ulcer or an increased tendency to bleed are not prescribed aspirin. Some patients on long-term, high dose aspirin therapy may experience some of the symptoms of acute overdose (see later), even if they are taking what for others is a safe therapeutic dose. There is evidence that aspirin use among children with a viral infection precipitates a serious life-threatening condition known as Reyes syndrome. For this reason aspirin is not recommended for use in children. The recommended dose for analgesic and antipyretic effect is one to two 325 mg tablets every four hours; no more than 12 tablets in 24 hours should be taken. For anti-inflammatory effect, that dose has to be increased to around two 500 mg tablets, usually every six hours. Just 75–150 mg/day is sufficient for anti-thrombotic effect.
Paracetamol
Absorption, metabolism and acute toxicity
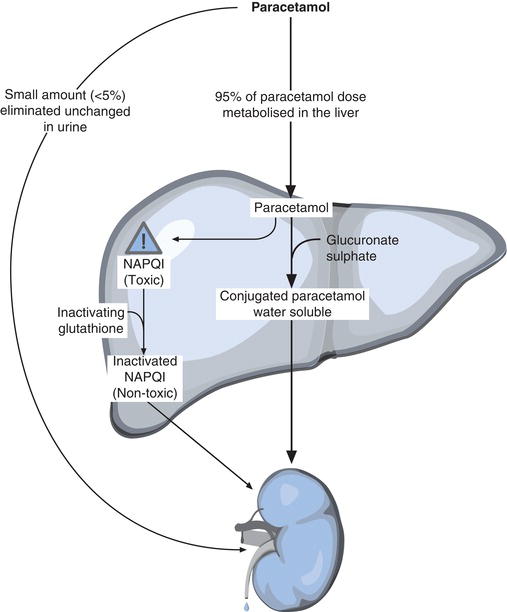
Paracetamol is quickly absorbed from the gastrointestinal tract to blood over a period of 30 minutes to 2 hours for a therapeutic dose. A small amount (up to 5%) is eliminated unchanged in urine, but the remainder is metabolised in the liver (Figure 13.1). Like most other drugs, paracetamol must be made more water soluble for appreciable elimination in urine. This is achieved in the liver, by synthetic conjugation (joining) of paracetamol with sulphate, glucuronate, glycine and phosphate. The resulting water-soluble conjugates of paracetamol are non-toxic. Around 90% of an ingested paracetamol dose is eliminated safely via urine in this way. The rest of the paracetamol (5–10% of an ingested dose) is oxidised in the liver, to a highly reactive and toxic free radical called N-acetyl-p-benzoquinone imine (NAPQI). It is NAPQI that is responsible for the toxic effects of paracetamol. At normal paracetamol dose the liver is able to inactivate this potentially damaging NAPQI by reaction with a substance called glutathione, which is synthesised in the liver. The product of the reaction between NAPQI and glutathione is non-toxic and is eliminated safely in urine and bile.
If there were no limit to the rate at which the liver could synthesise glutathione, then paracetamol would not be harmful, no matter how much had been taken, but unfortunately this is not the case. If recommended dose is exceeded the production of NAPQI increases, but the ability of the liver to synthesise the inactivating glutathione cannot keep pace, and NAPQI accumulates in liver cells, disrupting cellular mechanisms and eventually causing liver cell death (necrosis)8. Without prompt treatment, liver cell necrosis becomes extensive, leading to acute liver failure. At this stage liver transplantation may be the only treatment option.
A single dose of paracetamol of between 150–200 mg/kg is sufficient to cause liver cell damage in adults. This means that for an adult weighing an average 70 kg, just 10 g of paracetamol (i.e. 20 tablets) may be significant. Twelve grams (24 tablets) can be fatal and without treatment 25 g (50 tablets) is inevitably fatal. Certain drugs (notably the anticonvulsants phenytoin, carbamazepine and phenobarbitone) and alcohol result in increased production of the enzymes responsible for NAPQI production. Thus patients who have taken alcohol or these drugs are particularly susceptible to the toxic effects of paracetamol. Malnourished individuals are also more than normally susceptible because they have reduced liver glutathione reserves.
Signs and symptoms of acute overdose
The early symptoms of even potentially fatal paracetamol overdose are non-specific and unremarkable. For the first 24 hours, nausea and vomiting are the only symptoms and these may be absent; loss of consciousness is not an early feature. Signs and symptoms of extensive liver cell damage including jaundice, abdominal tenderness, continuing nausea and vomiting begin to develop in the 24–36 hours after overdose. Deterioration in liver function over the next few days leads to acute liver failure in the most severe cases. Drowsiness, and eventually coma (hepatic encephalopathy), is a feature of paracetamol overdose only at this late stage.
Principles of overdose treatment
So long as treatment is initiated early enough (i.e. within 12 hours of overdose), a complete recovery can be expected, even after a potentially fatal dose of paracetamol. Because of the speed at which paracetamol is absorbed from the intestine, efforts aimed at reducing absorption of paracetamol, including gastric administration of activated charcoal are only likely to be effective if begun within an hour or two of the overdose. The main treatment is early IV administration of the paracetamol antidote: N-acetylcysteine (NAC). In the body NAC is converted to glutathione, the substance required for inactivation of NAQI, the toxic metabolite of paracetamol. NAC is successful in preventing liver damage if administered within 12 hours of the overdose. Although less effective after 12 hours, some benefit can be gained by administration up to 24 hours, or even 72 hours in some cases, after overdose.
Aspirin
Absorption, metabolism and acute toxicity
Absorption of aspirin from the gastrointestinal tract is dependent on the amount and formulation of the drug. Therapeutic doses of regular (non-enteric coated) aspirin are absorbed rapidly within two hours. Larger quantities (overdose) of regular aspirin however inhibit gastric emptying with a resulting delay of up to six hours in intestinal absorption. Enteric coated formulations of aspirin are designed to be impervious to the acid content in the stomach and only begin to dissolve after arrival in the alkaline medium of the small intestine; such formulations may take up to 12 hours to be completely absorbed.
After absorption, aspirin is rapidly hydrolysed to salicylic acid (salicylate). This is the substance responsible for the acute toxicity as well as many of the therapeutic effects of aspirin. Before salicylate can be eliminated in urine it must first be conjugated with glycine to form salicyluric acid, or glucuronate to form phenolic glucuronides, but the enzymes for these detoxifying reactions become rapidly saturated at even therapeutic blood levels. As a consequence, salicylate accumulates in tissues in a dose dependent manner. Increased serum salicylate concentration is associated with increased stimulation of respiratory centre in the brain and thereby hyperventilation, which results in increased carbon dioxide elimination and respiratory alkalosis (Chapter 7). Salicylate at toxic levels has an effect on cellular metabolism which results in hyperpyrexia, sweating and abnormally high production of metabolic acids. Along with salicylate, itself an acid, these acids accumulate in blood causing metabolic acidosis. Salicylate causes increased permeability of the vasculature in the lungs, predisposing to the development of pulmonary oedema in salicylate overdose, particularly among smokers and the elderly. Finally salicylate can adversely affect normal control of blood glucose concentration; overdose of aspirin may result in hypoglycaemia (low blood glucose) or reduced levels of glucose in the brain, (neuroglycopaenia) despite normal blood glucose levels. Mild toxicity arises after a single dose of around 150 mg/kg body weight, whereas severe toxicity is associated with a single dose of greater than 500 mg/kg. For an adult of average weight (70 kg) then, just 20 tablets of 500 mg or 30 tablets of 325 mg are sufficient for mild toxicity.
Signs and symptoms of acute toxicity
Salicylate poisoning is more likely to be associated with early symptoms than paracetamol poisoning. Mild to moderate poisoning commonly causes nausea, vomiting and tinnitus, with loss of hearing. Patients are usually hyperventilating, hyperpyrexial and sweating. Dehydration secondary to vomiting, sweating and hyperventilation is usually a feature, particularly in severe poisoning. Blood gas analysis reveals disturbance of acid-base homeostasis (respiratory alkalosis or metabolic acidosis or combined disturbance). Acidaemia (low blood pH) is a poor prognostic sign because it enhances salicylate entry into tissue cells; entry of salicylate into brain cells causes additional neurological symptoms including confusion, delerium and extreme agitation. Loss of consciousness may occur, but is rare.
Principles of overdose treatment
There is no antidote for the treatment of aspirin overdose, as there is for paracetamol overdose. Treatment instead is based on three main objectives:
- Preventing further absorption of aspirin from the gastrointestinal tract.
- Increasing urinary elimination of salicylate.
- Correcting dehydration and deranged blood chemistry (acid-base and electrolyte disturbances, and hypoglycaemia if present).
The delay in intestinal absorption of high dose aspirin, especially enteric coated formulation means that oral administration of activated charcoal is more likely to be effective in reducing absorption than is the case in paracetamol overdose, although, of course, the sooner it is started, the more effective it will be. Urinary elimination of salicylate is increased if urine is made alkaline (pH >7.5) and urine output increased. This is achieved by administration of large volumes of sodium bicarbonate. Such treatment has the additional advantage of making blood more alkaline and thereby inhibiting the entry of salicylate into cells. Removal of salicylate from blood by haemodialysis or peritoneal dialysis may be considered in the most severe cases. Dextrose may be added to any IV fluid used to correct fluid and electrolyte disturbance since there is evidence that, even if blood glucose is normal, the brain is depleted of glucose in moderate to severe salicylate poisoning.
Laboratory measurement of salicylate and paracetamol
Patient preparation
No particular patient preparation is necessary.
Timing of sample
The time of blood sampling and the time of the overdose (if known) must be recorded on the accompanying request card. Because of varying rates of paracetamol absorption, it is impossible to accurately interpret a paracetamol result of a sample taken less than four hours after an overdose. Blood sampling should therefore be delayed until four hours have elapsed since paracetamol was taken. In all cases of salicylate poisoning it is useful to know the peak concentration. This can only be found by repeat sampling (every three hours) until a reduction in concentration is detected.
Sample requirements
Around 5 ml of venous blood is required for paracetamol and salicylate estimation. Analysis can be performed on either serum or plasma. If local policy is to use serum, blood must be collected into a plain (without additives) tube. If local policy is to use plasma, blood must be collected into a tube containing the anticoagulant, lithium heparin.
Transport of samples
The results of paracetamol and salicylate estimation are required for immediate patient management and therefore samples must be considered urgent and transported to the laboratory without delay.
Interpretation of paracetamol result
There seems to be no universal agreement about units of measurement for paracetamol; some laboratories report paracetamol in traditional units (mg/L or µg/ml); note that 1 mg/L = 1 µg/ml. Other laboratories use the SI unit of measurement (mmol/ml or mmol/L); note 1 mmol/ml = 1000 mmol/L.
To convert paracetamol concentration expressed in mg/L to paracetamol concentration expressed in mmol/L, divide by 151.
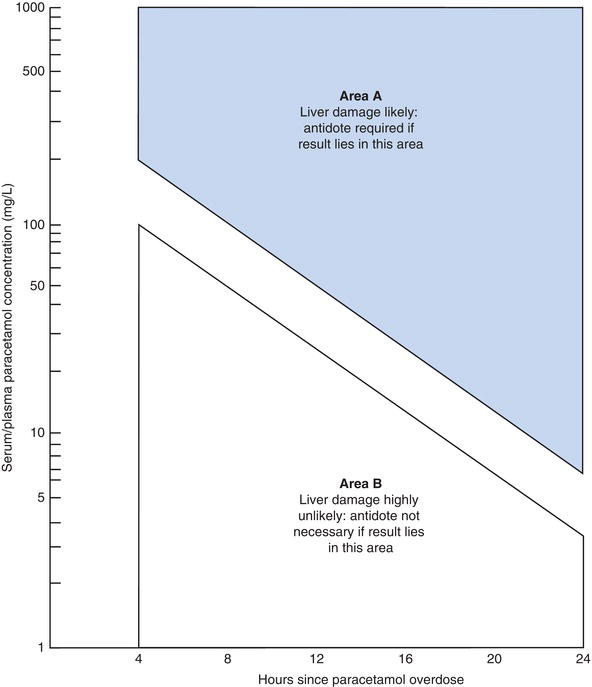
A potentially dangerous interpretation is possible if units are confused. Interpretation of paracetamol results depends crucially on knowing the approximate time of overdose. Figure 13.2 is a widely used nomogram, which allows assessment of the severity of overdose, and therefore risk of liver injury, based on the paracetamol concentration in relation to the time in hours since the overdose. For example, it can be seen from the graph in Figure 13.2 that a paracetamol concentration of 80 mg/L four hours after an overdose indicates that liver damage is extremely unlikely. However the same paracetamol result in a blood sample taken 12 hours after an overdose indicates that without antidote (N-acetylcysteine) treatment, severe, possibly fatal liver damage can be expected.
The graph highlights two important features of paracetamol toxicity. First it is not possible to accurately determine the severity of a paracetamol overdose, based on a serum paracetamol derived from blood sampled less than four hours after an overdose; a repeat sample would be advisable in these circumstances. Secondly any measurable amounts of paracetamol in serum 24 hours or later after an overdose indicate a poor prognosis especially as antidote treatment at this late stage is unlikely to be very effective in halting liver cell damage.
Interpretation of salicylate result
Again there is no consensus between laboratories concerning units of measurement. Most laboratories use either mg/L or mg/dl. Note: a salicylate concentration of 10 mg/dl = 100 mg/L.
Patients on long-term high dose aspirin therapy for chronic inflammatory conditions such as rheumatoid arthritis typically have a serum salicylate concentration of 25–35 mg/dl (i.e. 250–350 mg/L). These levels are rarely associated with any symptoms of acute toxicity. Mild to moderate toxicity occurs with peak salicylate concentration in the range 35–80 mg/dl (300–800 mg/L) whilst peak levels greater than 80 mg/dl (800 mg/ml) are associated with severe toxicity; around 5% of patients admitted to hospital with this level of toxicity do not survive. Serum salicylate levels provide an approximate and useful guide to the severity of a particular overdose but are not as reliable in this regard as serum paracetamol is in cases of paracetamol overdose. The clinical condition of the patient and the blood gas and electrolyte results are as important as plasma salicylate results for assessment of prognosis and clinical care planning. However some general points can be made. Nearly all symptomatic patients, even those with mild toxicity will be given activated charcoal. The likelihood that a patient will require alkalinisation of urine increases as the serum salicylate increases beyond 50 mg/dl. Finally haemodialyis is usually reserved for those patients whose serum salicylate is in excess of 80 mg/dl, although it may be considered the treatment of choice for all patients who have significant loss of renal function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree