- Anatomy and physiology of the pancreas
- Function of pancreatic and salivary amylase
- Serum amylase in diagnosis of acute pancreatitis
- Non-pancreatic causes of raised serum amylase
The measurement of amylase in blood plasma or serum is used almost exclusively in the investigation of patients with acute abdominal pain, a very common symptom especially among patients admitted urgently to hospital. Acute abdominal pain is almost invariably a major presenting symptom of common surgical emergencies like acute appendicitis, intestinal obstruction, perforated peptic ulcer and ruptured aortic aneurysm, so that rapid diagnosis, sometimes with the help of blood and urine tests, is important. A small proportion (around 3%) of patients whose principal symptom is acute abdominal pain will be suffering acute pancreatitis, a potentially life-threatening inflammatory disease of the pancreas. The serum amylase test is particularly useful in identifying these patients; a marked increase in serum amylase in a patient with acute abdominal pain is strongly suggestive of acute pancreatitis.
Normal physiology
The pancreas
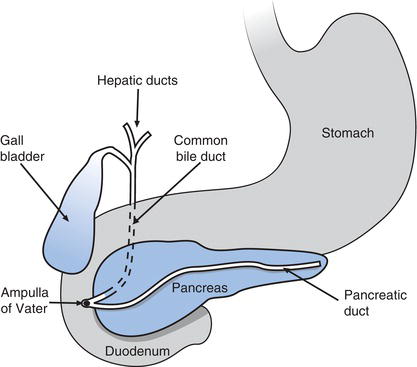
The pancreas is a soft, pale yellow/tan coloured organ, around 12–15 cm in length and weighing approximately 100 g. Its shape somewhat resembles a tadpole, with a just recognisable ‘head’, ‘body’ and ‘tail’. The organ lies transversely across the upper abdomen, with the ‘head’ positioned in the inner curve of the C shape formed by the first loop of the duodenum, the ‘body’ lying behind the stomach and the ‘tail’ extending from behind the stomach towards the spleen (Figure 12.1). The micro-anatomy of the pancreas reveals two sorts of functionally distinct tissue reflecting the dual (exocrine and endocrine) role of the pancreas. Around 90% of the pancreas comprises so called acinar (exocrine) tissue which is responsible for the production of pancreatic juice, a thin watery fluid required for intestinal digestion of dietary foodstuffs.
Pancreatic acinar cells are arranged like a bunch of grapes around a microscopical central tube or duct; acinar tissue comprises millions of these functional units. The ducts from each unit join, forming progressively larger ducts, which eventually drain all the pancreatic juice from the acinar cells into one large central duct called the duct of Wirsung, running the length of the pancreas. This central duct leaves the head of the pancreas and joins with the bile duct conveying bile from the gall bladder before joining with an opening in the duodenum called the hepato-pancreatic ampulla. Here, pancreatic juice (and bile) drains into the duodenum. Some people (around 20%) have a second pancreatic duct which drains pancreatic juice into the duodenum around 1 cm above the hepato-pancreatic ampulla.
Dispersed throughout the acinar tissue of the pancreas are highly vascularised islands of quite distinct (endocrine) cells that have no ductal connection with the duodenum. These are the islets of Langerhans, which are responsible for production of pancreatic hormones. There are three main cell types within the islets: alpha (α), beta (β) and delta (δ), each producing a specific hormone. β-cells, by far the most numerous, produce insulin; α-cells produce glucagon; and δ-cells produce somatostatin. These pancreatic hormones are released directly into the blood flowing through the islets and have their effect on cellular metabolism throughout the body. The function of insulin and glucagon in regulating blood glucose concentration is discussed in Chapter 3.
Pancreatic juice
The exocrine product of pancreatic acinar tissue, pancreatic juice, is a thin watery alkaline fluid (pH around 8) containing a mixture of many digestive enzymes, and electrolytes, most notably sodium, potassium, chloride and bicarbonate ions. With the exception of bicarbonate, electrolytes are present at similar concentration to that found in blood plasma; bicarbonate concentration of pancreatic juice is around four times higher. The relatively high bicarbonate concentration accounts for the alkaline reaction of pancreatic juice.
Pancreatic juice drains into the duodenum at the rate of 1500–3000 ml per day. Its principle function is to continue the digestion of food process in the small intestine, already begun in the mouth and stomach. The alkaline pH of pancreatic juice ensures that acid chyme (partially digested food) emptying from the stomach into the duodenum is rendered sufficiently alkaline (pH 7–7.5) for optimum pancreatic enzyme activity. The many digestive enzymes contained in pancreatic juice can be broadly divided into three groups according to the substrates in food on which they act: amylase for the digestion of carbohydrates, lipases for the digestion of fats and proteases for the digestion of proteins. Amylase and lipases are secreted in their active form, whereas proteases are secreted as proenzymes, capable of digesting proteins only after they have been activated within the duodenum. For example, trypsin, a protease present in intestine is derived from the inactive pancreatic pro-enzyme, trypsinogen. Pancreatic production and secretion of inactive pro-enzymes rather than their highly reactive product protects the pancreas from enzymic destruction.
The volume and content of pancreatic juice is controlled principally by hormonal pathways. Cholecystokinin (CCK), a gastrointestinal hormone released in response to gastric emptying of food into the duodenum, stimulates acinar cell production of digestive proenzymes. Secretin another gastrointestinal hormone promotes acinar cell production of bicarbonate. Neural pathways also affect pancreatic juice production. The vagal nerve, which is stimulated by the site, smell and thought of food, as well as the presence of food in the mouth, stimulates production of pancreatic juice. Release of pancreatic juice to the duodenum is controlled by a sphincter (the sphincter of Oddi) sited at the hepato-pancreatic ampulla. The sphincter opens when food is present in the duodenum. By a synergy of these and other subtle hormonal and neural mechanisms, the body is able to adjust the volume, content and release of pancreatic juice to suit its digestive requirement at the time.
Once pancreatic juice has performed its digestive function, around 99% of its water and electrolyte content is reabsorbed to the blood from the large intestine.
Amylase
Amylase is just one of several digestive enzymes delivered to the intestine in pancreatic juice. It is also secreted in saliva by three pairs of salivary glands in the mouth. Salivary and pancreatic amylase function only within the gastrointestinal tract where together, they are responsible for the breakdown of starch, the principal form of dietary carbohydrate. Starch is essentially many glucose molecules joined together. The product of amylase action on starch is a mixture of three sorts of molecule: the disaccharide maltose (2 molecules of glucose joined together); dextrin, a short chain of around eight glucose molecules; and some single molecules of glucose. Any glucose formed by the action of amylase on starch is absorbed to blood by active transport across the cells of the intestinal wall, but maltose and dextrin require further enzymic degradation by the intestinal enzymes, maltase and iso-maltase to single glucose molecules, before absorption can occur.
Like all enzymes, amylase will only work to maximum effect within a narrow pH range; for amylase the optimum pH is 7.1. Digestion of starch begins in the mouth with the action of salivary amylase, during the process of chewing. As soon as food reaches the acidic medium of the stomach (pH 2–3), salivary amylase action ceases. In practice, unless food is masticated in the mouth for a prolonged period, salivary amylase contributes little to overall starch digestion and most starch is broken down in the duodenum and jejenum by pancreatic amylase.
Normally a small amount of amylase circulates in blood plasma. Most of this is of pancreatic origin; some is derived from salivary glands. Amylase has no function in blood plasma and is present there only as a result of normal pancreatic and salivary cell turnover. By comparison with most other enzymes, amylase is a small molecule. Indeed it is small enough to pass through the glomeruli of the kidneys, so that it is one of very few plasma enzymes normally found in urine.
Laboratory measurement of serum amylase
Patient preparation
No particular patient preparation is necessary.
Sample requirements
Between 2 and 5 ml of venous blood is required.
The test is performed on either blood serum or blood plasma. If local policy is to use serum then blood must be collected into plain tube containing no anticoagulant. If local policy is to use plasma then blood must be collected into a tube containing an anticoagulant (usually heparin).
Timing of blood sampling and transport
Blood may be collected without reference to time. In many instances the result is required urgently so must be transported to the laboratory without delay. If the request is non-urgent the sample may be stored at room temperature before routine transport to the laboratory.
Interpretation of results
Approximate reference range serum (plasma) amylase: 50– 200 U/L
[Note: it is particularly important to use local reference range when interpreting enzyme results.]
Terms used
Hypoamylasaemia | serum amylase below normal. |
Hyperamylasaemia | serum amylase above normal. |
An abnormally low serum amylase has no known clinical significance apart from limited research that suggests it might be associated with higher than normal risk of cardiovascular disease for diabetic patients. Discussion will be confined to the interpretation of a raised serum amylase.
Causes of raised serum (plasma) amylase
Acute pancreatitis
Acute pancreatitis is a relatively common condition with an annual incidence of 22 per 100 000 population in the UK. Incidence has been increasing gradually over the past two decades, at a rate of 3% per year, with greatest increase among younger adults (11% increase per year for women aged less than 35 years and 5.6% annual increase for men aged 35–45 years).
There are two main causes: gall stones and alcohol abuse. Together these account for around 80% of cases. Excess alcohol consumption among young adults is thought to be responsible for increasing incidence in this age group.
A long list of much less common causes of acute pancreatitis include: trauma to the pancreas; over activity of the parathyroid gland (hyperparathyroidism); viral infections (e.g. mumps virus , Epstein-Barr virus, cytomegalovirus); parasitic worm infection; and marked increase in plasma triglycerides concentration. Very rarely acute pancreatitis occurs as a post-operative complication following upper abdominal surgery. Equally rare are those cases reported to be caused by prescribed drugs (e.g. thiazide diuretics, ACE inhibitors and steroids). Acute pancreatitis is a well documented but rare adverse effect of the invasive diagnostic procedure, endoscopic retrograde cholangiopancreatography (ERCP), occurring in around 4% of patients submitted for ERCP.
Acute pancreatitis is an acute inflammatory disease that is thought to result from the premature activation of proteolytic (protein splitting) enzymes. Activation of these enzymes within pancreas leads to a process of ‘autodigestion’, in essence self-destruction of the pancreas. Activation of trypsinogen to trypsin within acinar cells is widely proposed as the initiating event, though it remains unclear precisely how alcohol abuse or gall stone disease leads to this initiating event or promotes the autodigestion and inflammation that flows from it. Obstruction to the flow of pancreatic juice by gall stones transiently lodged in the hepato-pancreatic ampulla is significant among those with gallstone related acute pancreatitis.
The cardinal symptom is sudden onset of severe upper abdominal pain, which often radiates to the back. Vomiting and pyrexia is common. The course of acute pancreatitis is variable. In the majority (75–80%) of patients, the inflammation is self-limiting, confined to the pancreas and resolves over a period of a few days to a week with no serious long-term consequences. However, this is not the case for the 20–25% of patients who suffer severe acute pancreatitis. This is a life threatening condition in which the local inflammation leads to systemic inflammatory response syndrome (SIRS) with high risk of sepsis and multiple organ failure. Complications of severe disease include overwhelming infection consequent on massive tissue necrosis (which may not be confined to pancreas), acute respiratory distress syndrome, jaundice, anaemia, hyperglycaemia, hypocalaemia, disseminated intravascular coagulation and haemorrhage. Early (within 12–24 hours) admission to intensive care is necessary and usually lifesaving but, despite intensive care, around 20–30% of patients with severe acute pancreatitis die, usually due to overwhelming infection and associated multiple organ failure.
Damage to acinar cells, the central pathological feature of acute pancreatitis, results in a sudden and massive increase in release of pancreatic enzymes into the blood stream; among these is amylase. This increase in serum amylase has no clinical consequences of itself, that is no signs or symptoms of acute pancreatitis can be attributed to an increase in serum amylase. It does however provide a marker in the blood, of damage to the pancreas. Serum amylase begins to rise 2–12 hours after the onset of symptoms and remains elevated for three to five days. A serum amylase level between three and five times the upper limit of normal (i.e. 800–1000 U/L) in a patient with acute abdominal pain is widely considered to be strongly suggestive of acute pancreatitis. The probability that acute pain is due to pancreatitis increases as amylase level rises above 1000 U/L. A minority of patients with acute pancreatitis do not show such a marked increase so that a serum amylase less than 800 U/L cannot be used to exclude the diagnosis and, rarely, serum amylase may be within normal limits in a patient with acute pancreatitis. It might be assumed that the higher the serum amylase, the more severe the pancreatitis; that is not the case. In fact no prognostic information can be derived from measurement of serum amylase at the time of diagnosis. However failure of amylase to return to normal following an acute attack suggests the presence of a pancreatic pseudocyst (a late complication of acute pancreatitis).
Chronic pancreatitis
Natural repair of the damage caused by inflammation of acute pancreatitis leaves the pancreas of someone who has recovered from acute pancreatitis, functioning normally. By contrast chronic pancreatitis is a chronic inflammation in which damage to the pancreas is slow but irreversibly progressive. The most common cause is long-term alcohol abuse. It may be a feature of haemochromatosis, a disease of iron overload in which excess iron is deposited in the pancreas and other organs. Continuous or at least recurrent abdominal pain is the cardinal symptom of chronic pancreatitis. Long term the condition results in malabsorption of food and consequent weight loss, due to failure of pancreatic enzyme production, and diabetes as a result of islet cell damage. Serum amylase may be slightly raised in the early stages but as acinar cell production of digestive enzymes (including amylase) becomes increasingly compromised, serum amylase falls to normal or even to levels below normal. Since amylase may be raised, normal or reduced, the test serves no useful purpose in the diagnosis of chronic pancreatitis.
Cancer of the pancreas
Apart from acute and chronic pancreatitis, cancer of the pancreas is the only other significant disease of the pancreas. Serum amylase is either marginally raised or normal; the test is not useful for the diagnosis of cancer of the pancreas.
Non pancreatic disease
Serum amylase may be mildly to moderately raised (i.e. rarely greater than 800 U/L) in some non-pancreatic disorders, for example, perforation of peptic ulcer, intestinal obstruction and gall bladder disease (acute cholecystitis). All these conditions are usually associated with acute abdominal pain so that a patient with a mildly raised serum amylase in association with acute abdominal pain is not necessarily suffering acute pancreatitis. Abdominal trauma, not necessarily involving the pancreas, may result in an increased amylase; a significant minority of patients who receive abdominal surgery have a transient rise during the post-operative period.
Amylase is cleared from the blood by the kidneys, so that patients in acute or chronic renal failure typically have slight to moderate increases in serum amylase. A moderate to marked increase is often a feature of diabetic ketoacidosis, a condition discussed in Chapter 3. Disease of, or damage to, the parotid glands where salivary amylase is produced may result in an increase in serum amylase. Examples include infection with the mumps virus, maxillofacial surgery and parotid gland irradiation.
Finally, raised serum amylase is a feature of a rare and entirely benign condition called macroamylasaemia, in which amylase circulates in serum in the form of macromolecular aggregates of amylase or bound to serum proteins. Because they are so large these macromelcules cannot pass across the glomerular membrane of the kidneys and are not excreted in urine; instead they accumulate in serum.
Table 12.1 provides a summary of the principle causes of raised serum amylase.
Table 12.1 Principal causes of raised serum/plasma amylase.
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree