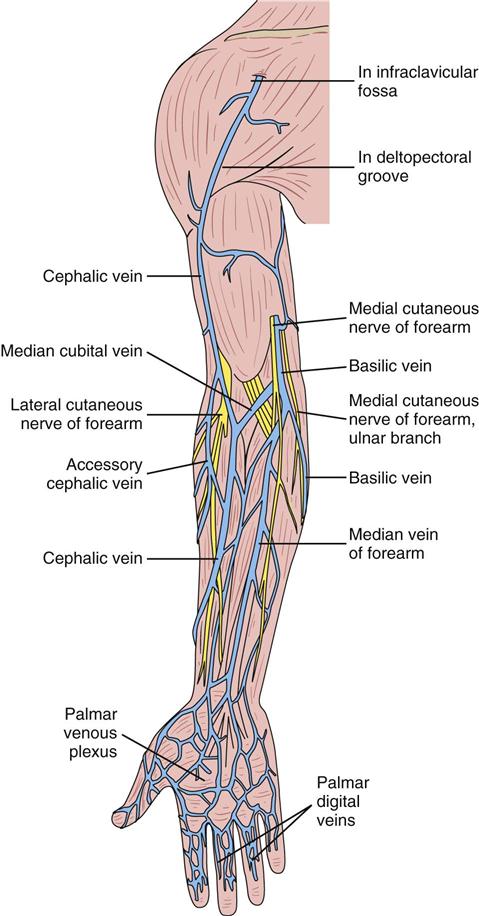
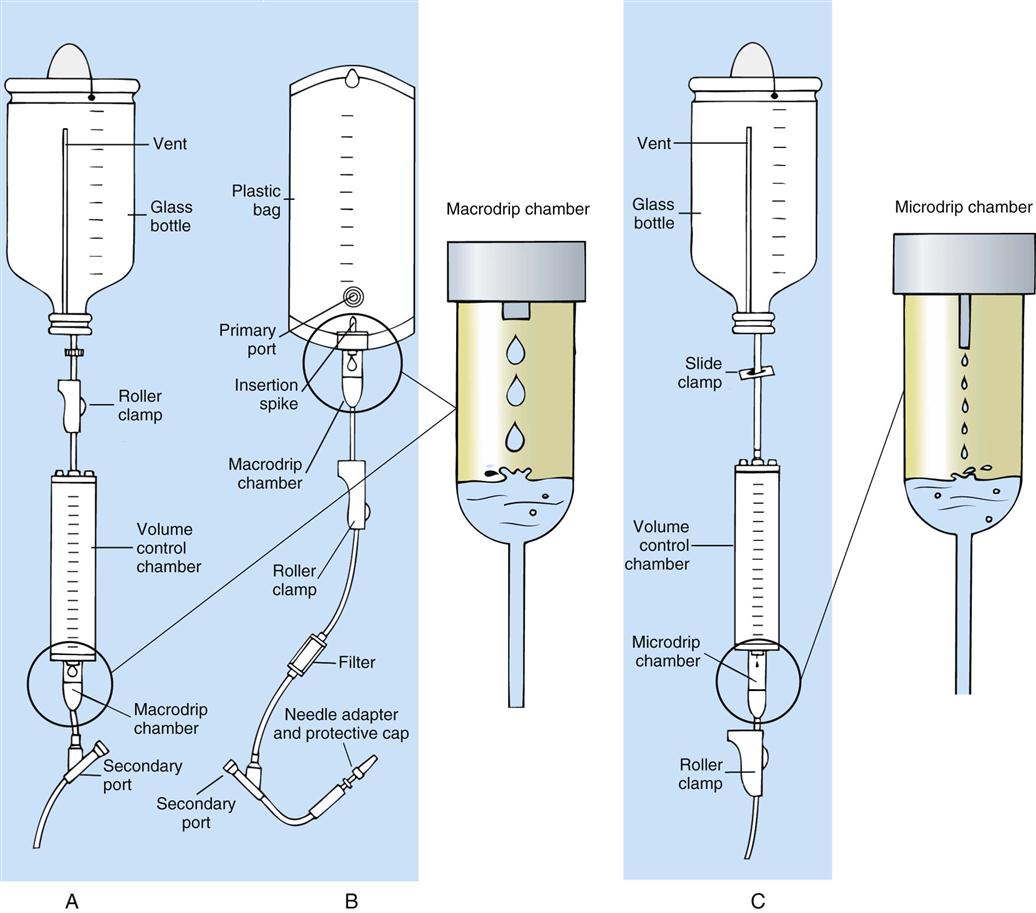
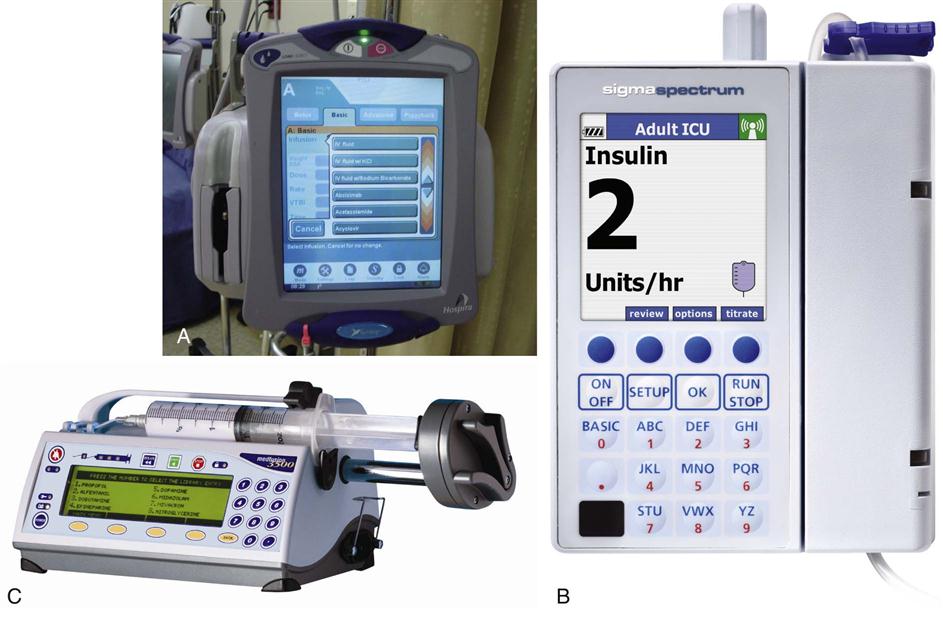
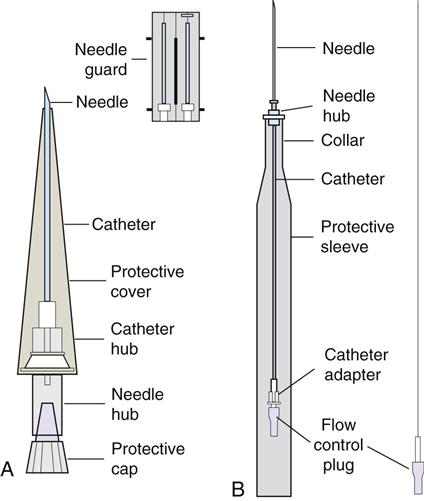
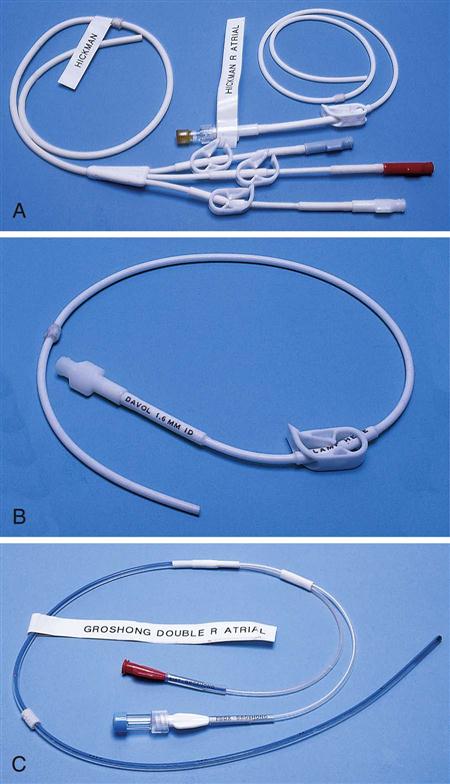
IV administration set (p. 169) nonvolumetric IV controllers ( volumetric IV controllers ( peripheral devices ( implantable venous infusion ports ( winged, butterfly, or scalp needles ( over-the-needle catheters (p. 172) saline lock, heparin lock, or medlock ( in-the-needle catheters (p. 173) peripherally inserted central venous catheters (PICCs) ( tunneled central venous catheters ( The term intravenous (IV) administration refers to the introduction of fluids directly into the venous bloodstream. The advantages of this technique are that large volumes of fluids can be rapidly administered into the vein and that there is usually less resultant irritation. IV administration is the most rapid of all parenteral routes, because it bypasses all barriers to drug absorption. Drugs may be given by direct injection with a needle in the vein, but they are more commonly administered intermittently or by continuous infusion through an established peripheral or central IV line. An advantage of IV administration as compared with other forms of parenteral administration is that it is generally more comfortable for the patient, especially when several doses of medications must be administered daily. However, disadvantages to the IV route include nurses needing the skill to establish and maintain an IV site. In addition, the patient tends to be less mobile with IV administration, and there is a greater possibility for infection as well as for severe adverse reactions to the drug. The term IV nursing is defined by the Infusion Nurses Society as the use of the nursing process as it relates to technology and application, fluids and electrolytes, pharmacology, infection control, pediatrics, transfusion therapy, oncology, parenteral nutrition, and quality assurance. Before a nurse is eligible to perform IV therapy procedures, he or she must meet the institutional guidelines that pertain to infusion therapy. Some hospitals use infusion therapy teams, but many now assign the responsibility for infusion therapy to nurses with earned credentials. The nurse who is performing venipuncture and infusion therapy must be well versed in the techniques described previously in the definition of IV nursing. All IV therapy requires a written order from a health care provider that is dated and that specifies the type of solution or medication to be administered, the dosage, and the rate and frequency of administration. Many institutions nationwide have acknowledged the value of the licensed practical nurse/licensed vocational nurse (LPN/LVN) as a member of the IV team. Most state laws recognize the role of the LPN/LVN in IV therapy but delegate the scope of practice to be defined in the policies and procedures of individual practice sites. The nurse should check with his or her particular state board of nursing to determine the current guidelines and education requirements. In general, LPN/LVN responsibilities do not include the administration of IV medication, blood products, or antineoplastic agents. Before any nurse administers IV therapy, he or she should ask the following questions: Certification in infusion therapy is available for registered nurses by taking a written examination offered by the Infusion Nurses Certification Corporation. The nurse can use the initials “CRNI” (Certified Registered Nurse Infusion) in his or her title after passing the certification process for a period of 3 years. The nurse must meet the established criteria for recertification at 3-year intervals. Clinical sites and other local agencies also offer courses in infusion therapy at both introductory and advanced levels. The state board that governs nursing can provide information about the requirements for registered and licensed practical nurses to perform infusion therapy. The Infusion Nurses Society, a professional nursing organization, publishes the Infusion Nursing Standards of Practice; the Centers for Disease Control and Prevention (CDC) publishes recommendations for infection control related to infusion therapy. These are excellent sources to consult when reviewing standards for IV therapy. An IV administration set is an apparatus that connects a large volume of parenteral solution with the IV access device in the patient’s vein. All sets (Figure 12-1) have an insertion spike, a drip chamber, plastic tubing with a rate-control clamp, a rubber injection portal, a needle adapter, and a protective cap over the needle adapter. Depending on the manufacturer, the sets are available in a variety of styles (e.g., different volumes and sizes of drip chamber, “piggyback” portals, filters, styles of control clamps [see Figure 12-1]). The type of system used by a particular hospital is usually determined by the manufacturer of the IV solutions used by the institution. Each manufacturer makes adapters to fit a specific type of plastic or glass large-volume solution container. A crucial point to remember about administration sets is that the drops delivered by drip chambers vary among manufacturers. Macrodrip chambers (see Figure 12-1, A and B) provide 10, 15, or 20 drops/mL of solution, whereas microdrip chambers (see Figure 12-1, C) deliver 60 drops/mL of solution (see p. 170). Microdrip administration sets are used when a small volume of fluid is being administered, particularly when accuracy of volume administration is indicated (e.g., with neonatal and pediatric populations; for those patients with fluid volume concerns). Volume-control chambers (see Figure 12-1, A and C) are also used as a safety factor to limit the volume administered. In many clinical settings, microdrip sets are used for all volumes of IV fluid ordered that are administered at less than 100 mL/hr. It is essential to read the label on the box before opening it to ensure that the correct IV administration set has been selected. The nurse must be able to calculate the flow rate for the IV solution when hanging IV solutions that will flow by gravity. The flow rate is calculated in drops per minute and the nurse will time an IV fluid drip by counting the drops for one minute. A typical flow rate problem would be as follows: How fast will the nurse time the rate of the IV if the order reads, “Infuse 200 mL of D5W over 2 hours”? When calculating the drips per minute, the formula used is related to the drip chamber according to the manufacturer. Using 20 drops/mL, the problem will be set up by starting with the known, then multiplying the number of hours by the number of minutes in an hour, and then multiplying by the drip factor for the tubing: The quickest way to time 33 drops per minute would be to time the drips for 30 seconds; if 16 drops fall during that time, the rate will be set. Keep in mind that the use of IV infusion pumps is preferred because the gravity method tends to be inaccurate over time. The flow rates that are used for infusion pumps are generally in mL per hour. In the problem given in the previous paragraph, the infusion pump would be set at 100 mL/hour for a total infusion volume of 200 mL; see Chapter 6 for more examples. A large variety of connector and access devices are available for various components of infusion therapy. The nurse must become familiar with the IV access systems and the terms used for these systems at the clinical site. Knowing which parts of the system are clean and which parts are to remain sterile is crucial for the provision of safe IV therapy. Devices have been developed to ensure safety and to accurately control the infusion rates of IV solutions. Precise rates are important for certain therapeutic effects (e.g., with a continuous heparin infusion for anticoagulation) or when monitoring the administration of medications to prevent toxicity (e.g., nephrotoxicity, ototoxicity). Infusion devices can be classified as controllers, pumps, and syringe pumps (Figure 12-2). The basic IV infusion through a peripheral IV access site depends on gravity for administration. The IV container must be 24 to 36 inches above the IV site so that the pressure of the infusion solution in the tube is greater than the resistance (e.g., back pressure) from the vein, thereby allowing the infusion to flow into the vein. If the height changes significantly because the patient changes position or if the fluid volume remaining in the IV container is minimal, the IV infusion will slow down or stop. The simplest rate controllers are the roller-and-slide clamps (Figure 12-3) that accompany IV administration sets. These constricting devices regulate flow by being adjusted while the nurse counts the number of drops per minute that pass through the drip chamber. (It is important to remember that macrodrip chambers form 10 to 20 drops/mL of solution, whereas microdrip chambers form 60 drops/mL of solution.) Electronic control devices can be categorized as nonvolumetric or volumetric control devices. The nonvolumetric IV controllers monitor only the gravity infusion rate by counting the drops that drip through the chamber. These devices protect the patient by sounding an alarm if the clamp slips and results in an inadvertent-free flow. The controllers are also designed to set off an alarm if the number of drops per minute slows down or stops because of patient position changes, air in the tubing, or low solution volume. Nonvolumetric controllers have no moving parts, so maintenance costs are modest as compared with pumps; however, nonvolumetric controllers are less accurate with viscous solutions (e.g., blood products). The volumetric IV controllers are actually pumps that apply external pressure to the administration set tubing to squeeze the solution through the tubing at a specific rate (e.g., a certain number of mL/min or mL/hr). Volumetric control devices can be programmed for a specific volume over time and are much more accurate than the nonvolumetric controllers. These pumps also have an alarm system that sounds if there is resistance in the IV line caused by a developing occlusion as a result of thrombus formation or a kink in the administration set line caused by patient movement. Disadvantages of pumps are the cost of the equipment and of the training of personnel, the cost of maintenance, more equipment needed at the bedside, and the potential for more serious infiltration than occurs with gravity flow administration. Syringe pumps hold a prefilled syringe and apply positive pressure to the plunger to deliver a specific volume of medicine over a set time. Syringe pumps are more commonly used when small volumes need to be administered. Examples of small syringe pumps are those that continually infuse insulin into the subcutaneous tissue of patients with diabetes mellitus as well as patient-controlled analgesia pumps, which allow patients who are receiving pain medications to administer continual infusions and intermittent boluses of the medicine for comfort. Volumetric pumps and syringe pumps have become sophisticated and much safer. These pumps have alarm systems and automatic stopping capabilities; they can be preprogrammed to prevent calculation errors, and they can be monitored from a distant site with the use of a modem. Syringe pumps are easy to use, and their use is taught to patients needing home infusion therapy, when patients self-administer medications through an implanted infusion port. It is important that the nurse become familiar with the specific devices that are used in the clinical setting for safe and efficient patient care. IV access devices are often subdivided into four groups on the basis of the location of the terminal tip of the access device: (1) peripheral devices are for short-term use in peripheral veins in the hand or forearm; (2) midline catheters are for use over 2 to 4 weeks, and they are inserted into intermediate-sized veins and advanced into larger vessels; (3) central devices are inserted into intermediate-sized vessels and advanced into central veins, where the tip of the catheter typically will be in the superior vena cava to allow for maximal mixing with large volumes of blood; and (4) implantable venous infusion ports, which are placed into central veins for long-term therapy. All needles—if they are long enough—may be used to administer medications or fluids intravenously. However, special equipment has been designed for this purpose. Winged needles, which are also known as butterfly or scalp needles, are short, sharp-tipped needles (Figure 12-4) that were originally designed for venipuncture of small veins in infants and for geriatric use. These needles are available in sizes that range from 17 to 29 gauge, and they have been designed to minimize tissue injury during insertion. The winged area is pinched together to form a handle while the needle is being inserted, and it is then laid flat against the skin to form a base that can be anchored with tape. Two types of these needles are now available: one with a short length of plastic tubing and a permanently attached resealable injection port and one with a variable length of plastic tubing with a female Luer adapter for the attachment of a syringe or an administration set (see Figure 12-4). The patency of the needle is maintained with the use of a heparin or saline flush routine in accordance with facility policy. Over-the-needle catheters, which are also known as short peripheral venous catheters, are recommended for routine peripheral infusion therapy. The needles are stainless steel and coated with a Teflon-like plastic catheter (Figure 12-5, A). After the needle penetrates the vein in the hand or forearm, the catheter is advanced into the vein, and the metal needle is removed, thereby leaving the plastic catheter in place. An IV administration set is then attached to the catheter for continuous infusion. This unit is used when IV therapy is expected to continue for a few days. The rationale for the use of the plastic catheter is that it does not have a sharp tip that could cause venous irritation and extravasation. When a patient no longer requires IV fluid therapy but venous access is still needed for medication administration, an extension tube with an injection port is attached to the catheter, and the IV fluid is discontinued. The IV access device is then referred to at different clinical sites as a saline lock, a heparin lock, or a medlock. The term heparin lock originated when both saline and heparin were used to flush the short peripheral venous catheter to prevent blockage as a result of clot formation. Research indicates that normal saline flushing is sufficient to prevent clotting and to maintain the peripheral catheter integrity, so a more appropriate term is saline lock or medlock (e.g., medication lock). Generally, peripheral catheters should be changed every 72 to 96 hours to prevent infection and phlebitis. Blood samples should not be drawn from peripheral catheters. If sites for venous access are limited and no evidence of infection is present, peripheral venous catheters can remain in place, although the patient and the insertion site should be monitored closely for signs and symptoms of phlebitis, infiltration, and infection. The CDC recommends that peripheral catheters not be changed for pediatric patients unless this is clinically indicated. In-the-needle catheters make use of large-bore needles for venipuncture (see Figure 12-5, B). A 4- to 6-inch sterile, smaller-gauge, plastic catheter is then advanced through the needle into the vein. The needle is withdrawn, and the skin forms a seal around the plastic catheter. The IV administration set is attached directly to the plastic catheter. In-the-needle catheters are seldom used today for peripheral IVs because of the risk of shearing the through-the-needle catheter. Midline access catheters are selected for use if it is anticipated that IV access will be needed for 7 days or more. These catheters are often left in place for 2 to 4 weeks. Midline catheters are flexible and 3 to 8 inches long, and they are inserted at the antecubital fossa into the cephalic or basilic vein and advanced to the distal subclavian vein. They do not enter the superior vena cava. Midline catheters appear to be associated with lower rates of phlebitis than short peripheral catheters; they have a lower rate of infection, and they cost less than central venous catheters. The CDC recommends the replacement of the catheter and the rotation of the injection site no more frequently than every 72 to 96 hours, but the organization does not provide recommendations regarding the maximum length of time that the catheter may remain in place. Many institutions require that the health care provider write an order that indicates that the IV may be left in place for more than 72 hours. Midline catheters are used for continuous access, repeated access, or IV solutions with high flow rates. This type of catheter needs to be flushed with saline and heparin solution after each use or at least once daily if it is not in use. Blood should not be drawn through this catheter. Central IV access devices, which are also known as indwelling catheters, are used in the following situations: when the purpose of therapy dictates their use (e.g., large volumes of medication, for irritating medicine such as chemotherapy, or when hypertonic solutions such as total parenteral nutrition are to be infused); when peripheral sites have been exhausted as a result of repeated use or the condition of the veins for access is poor; when long-term or home therapy is required; or when emergency conditions mandate adequate vascular access. The central venous sites that are most commonly used for central venous catheters are the subclavian and jugular veins. When the upper body veins are not acceptable, the femoral veins may be accessed for short-term or emergency use. A physician can also elect to perform a venisection or a cutdown to insert this type of catheter into the basilic or cephalic veins in the antecubital fossa. Three types of devices based on the placement of the catheter’s proximal tip are routinely used for central catheters: peripheral devices, tunneled devices, and implantable devices. Peripherally inserted central venous catheters (PICCs) are inserted into the superior vena cava or just outside the right atrium by way of the cephalic or basilar veins of the antecubital space, thereby providing an alternative to subclavian or jugular venous catheterization. PICCs are available in sizes that range from 14 to 28 gauge, with various lengths, thereby making them available for pediatric use. The catheter itself can have an open tip or a valved (Groshong) tip, and it comes with a single or double lumen. The PICC line has the advantage of ease of insertion, because the procedure can be performed at the bedside by a qualified nurse. PICCs are associated with fewer mechanical complications (e.g., thrombosis, hemothorax); they cost less than other central venous catheters, they are easier to maintain than short peripheral catheters (because there is less frequent infiltration and phlebitis), and they require less frequent site rotation. PICC lines routinely remain in place for 1 to 3 months, but they can last for 1 year or more if they are cared for properly. When the device is not in use, the IV is disconnected, and the catheter is flushed and capped. The line should be flushed with a saline–heparin solution after every use or daily, if not used, in accordance with institutional policy. Tunneled central venous catheters are surgically placed during an outpatient procedure, with the patient under local anesthesia. The terminal tip of the catheter is inserted through an incision and into the subclavian vein, and it is then advanced to the superior vena cava. The proximal end of the catheter is tunneled about 6 inches away under the skin on the chest to exit near a nipple. A Dacron cuff is often placed around the catheter under the skin, which anchors the catheter and forms a seal around the catheter as the skin heals, thereby helping to keep the tunnel sterile. Three types of catheters that are frequently used are the Hickman, Broviac, and Groshong catheters (Figure 12-6). The Broviac catheter is a single-lumen catheter with a larger external diameter and a standard end hole. The Hickman catheter is larger in diameter than the Broviac catheter, but it contains two or three lumens; it also has a standard end hole. When they are not in use, both of these catheters are clamped to prevent contamination, clotting, and air embolism. These catheters must also be flushed with a saline–heparin solution after every medication administration or at least once daily if they are not in use. The Groshong catheter contains one to three lumens, and each one has a rounded valved tip. The Groshong valve opens inward for blood sampling and outward for infusion, but it remains closed when it is not in use. Because the valve remains closed when it is not in use, it seals the fluid inside the catheter and prevents it from coming into contact with the patient’s blood. Thus, weekly flushing with saline solution is all that is required to keep the catheter patent. The valve also eliminates the need for routine clamping of the catheter, although it should remain capped when it is not being used. Implantable infusion ports (e.g., Infus-A-Port, Port-A-Cath) are used when long-term therapy is required and intermittent accessing of the central vein is required for the administration of IV fluids, medications, total parenteral nutrition (TPN), chemotherapy, and blood products. The implantable devices are similar to tunneled devices with regard to placement; however, the proximal end of the single- or double-lumen catheter is attached to a single- or double-lumen access port (Figure 12-7) and then implanted and sutured into a subcutaneous pocket in the chest area or the upper arm. The double ports are designed to allow for the administration of two IV solutions, two IV medications, or one of each simultaneously. One port can also be reserved for drawing blood samples. The ports contain a self-sealing silicone rubber septum that has been specifically designed for repeated injections over an extended period of time. A special noncoring, 90-degree–angle Huber needle is used to penetrate the skin and the septum of the implanted device to minimize damage to the self-sealing septum. To prolong the life of the septum, only the smallest-gauge noncoring needles should be used. The chest port is estimated to withstand up to 2000 punctures, whereas the arm port has an estimated life of 1000 punctures. An implanted central venous access catheter may remain in place for more than 1 year, and it requires only a saline–heparin solution flush after every access or once monthly. Because the entire port and catheter are under the skin, there is no daily maintenance needed, although the site should be monitored visually on a regular basis to check for swelling, redness, or drainage. This type of central venous catheter gives the patient the greatest flexibility in terms of daily activities and exercise, including swimming; however, contact sports should be avoided. All central venous access devices require postinsertion radiography to verify the location of the device and to check for the presence of a pneumothorax for catheters that are tunneled on the chest. The CDC recommends that central venous catheters not be routinely replaced to prevent catheter-related infection. Review Chapter 10 for information about the use of ampules, vials, and Mix-O-Vials. All parenteral drug dose forms are packaged so that the drug is sterile and ready for reconstitution (if needed) and administration. Under normal and healthy conditions, the body loses water and electrolytes daily through urine, perspiration, and feces. Fluids are replenished as a result of the absorption of water in the gastrointestinal tract from the liquids and foods that are consumed. However, as a result of many different disease states (e.g., vomiting, diarrhea, gastrointestinal suctioning, hemorrhage, drainage from a wound, decreased intake, nausea, anorexia, fever, excess loss from disease [e.g., uncontrolled diabetes mellitus, diabetes insipidus]), patients are unable to ingest sufficient quantities of fluid and electrolytes to offset these losses. When this happens, the IV infusion of solutions may be necessary for replacement. See a medical–surgical nursing textbook for more information about patient assessments for deficient fluid volume. Intravenous (IV) solutions (Box 12-1) consist of water (e.g., a solvent) that contains one or more types of dissolved particles (e.g., solutes). The solutes that are most commonly dissolved in IV solutions are sodium chloride, dextrose, and potassium chloride. The solutes that dissolve in water and dissociate into ion particles (e.g., Na+ and Cl−, K+, and Cl−) are called electrolytes, because these ions give water the ability to conduct electricity. Total parenteral solutions contain all the electrolytes necessary in addition to enough carbohydrates (usually dextrose), amino acids, and fatty acids to sustain life. From a physiologic standpoint, the water in the body is distributed among three compartments: (1) the intravascular compartment (e.g., the arteries, veins, and capillaries); (2) the intracellular compartment; and (3) the interstitial compartment (e.g., the spaces between the cells that are outside of the vascular compartment; Figure 12-8). The extracellular compartment is composed of the intravascular and interstitial compartments, and it contains about one third of the total body water, whereas the intracellular compartment contains about two thirds of the total body water. The spontaneous movement of water across the intravascular compartment capillary membranes to the interstitial spaces and across the cell membranes and back to the intravascular capillary space is called osmosis. The water moves from an area of high concentration of water (e.g., of low electrolyte concentration) to an area of low water concentration (e.g., of high electrolyte concentration). The electrolyte and protein contents of each compartment are what draw water into the compartment until there is equilibrium between compartments. The force caused by the electrolytes and proteins is called osmotic pressure. The concentration of the dissolved particles in each compartment is known as the osmolality. Normal blood serum osmolality is 295 to 310 milliosmoles per liter (mOsm/L). Because IV solutions also contain dissolved particles, they also have an osmolality. If the IV solution and the blood have approximately the same osmolality, then the solution is said to be isotonic. Solutions that have fewer dissolved particles than the blood are considered to be hypotonic, and those with a higher concentration of dissolved particles are thought of as hypertonic. A 0.9% solution of sodium chloride, which is also known as normal saline (NS) or physiologic saline, is an isotonic solution with an osmolality of 308 mOsm/L. See Table 12-1 for commonly used IV solutions, their electrolyte concentrations, and their osmolalities. Those solutions with osmolalities below 270 mOsm/L are hypotonic; those 270 to 310 mOsm/L are isotonic; those with values of more than 310 mOsm/L are hypertonic. Table 12-1 Intravenous Solutions, Electrolyte Concentrations, and Osmolality *Also contains the following: K+ = 4 mEq/L; lactate = 28 mEq/L; Ca2+ = 3 mEq/L. Isotonic solutions (e.g., 0.9% sodium chloride, lactated Ringer’s) are ideal replacement fluids for the patient with an intravascular fluid deficit (e.g., acute blood loss as a result of hemorrhage, gastrointestinal bleeding, or trauma). This type of fluid is used for hypovolemic, hypotensive patients to increase vascular volume to support blood pressure; however, these patients must be monitored for fluid overload (potential pulmonary edema), especially if the patient has congestive heart failure. Another isotonic solution, dextrose 5% with 0.2% sodium chloride (D5/0.2 NS), is a standard solution for maintaining hydration and electrolytes (e.g., potassium chloride), administering continuous infusion IV medications, and to keep open (TKO) IV therapy for the intermittent administration of medications. D5/0.2 NS solutions are infused as isotonic solutions, but they rapidly become hypotonic solutions as the dextrose is metabolized. Therefore, D5/0.2 NS solutions—even though they are initially isotonic—should not be used to maintain vascular volume in a patient who is hypovolemic and hypotensive. Hypotonic solutions (e.g., 0.2% or 0.45% sodium chloride) have lower osmolality than serum. This type of solution contains fewer electrolytes and more free water, so the water is rapidly pulled from the vascular compartment into the interstitial and intracellular fluid compartments. Although these solutions are useful in conditions of cellular dehydration, administering them too rapidly may cause a sudden shift of fluids being drawn from the intravascular space into the other compartments. Hypertonic solutions have an osmolality that is higher than that of the serum. Although hypotonic and isotonic solutions are used in particular situations because of their tonicity, hypertonic solutions are rarely used in this way, because hypertonic solutions have the potential to pull fluid from the intracellular and interstitial compartments into the intravascular compartment, thereby causing cellular dehydration and vascular volume overload. Hypertonic solutions also have the disadvantage of causing phlebitis and venous spasm, with infiltration and extravasation occurring in the peripheral veins. In general, solutions with osmolalities of more than approximately 600 to 700 mOsm/L should not be administered in peripheral veins. Hypertonic solutions (e.g., parenteral nutrition solutions) must be administered through central infusion lines, where the solution can be rapidly diluted by large volumes of rapidly flowing blood (e.g., in the superior vena cava near the entrance to the right atrium). IV solutions are available in both plastic and glass containers in a variety of types and concentrations (see Table 12-1 and Box 12-1) and in volumes that range from 100 to 2000 mL. Both the glass and plastic containers are vacuum sealed. The glass bottles are sealed with a hard rubber stopper and then a metal disk, and this is followed by a metal cap. Right before use, the metal cap and disk are removed, thereby exposing the hard rubber stopper. The insertion spike of the IV administration set is pushed into a specifically marked area on the rubber stopper. Some brands also have another opening in the rubber stopper that serves as an air vent (see Figure 12-1, A and C). As the solution runs out of the container, it is replaced with air. Other brands make use of a flexible plastic container (see Figure 12-1, B). As the solution runs out of the bag, the flexible container collapses. Plastic bags are somewhat different in that the entire bag and solution are sealed inside another plastic bag for removal just before administration. When the insertion spike is forced into the specially marked portal, an internal seal is broken, which allows the solution to flow into the tubing. Some medicines (e.g., antibiotics) are administered by intermittent infusion through an apparatus known as a tandem setup, piggyback, or IV rider (Figure 12-9). These medicines are given by a setup that is hung in tandem and connected with the primary setup. The secondary setup may consist of a drug infusion from a small volume of fluid in a small bag or bottle (≤250 mL; see Figure 12-9) or from a volume-control set (also known as a Volutrol or a Buretrol; see Figure 12-1, A and C). A volume-control set is composed of a calibrated chamber that is hung under the primary IV solution container and that can provide the necessary 50 to 250 mL of diluent per dose of drug. Most intermittent diluted drug infusions are infused over 20 to 60 minutes. Medications for IV administration are available in ampules, vials, prefilled syringes, and large-volume IV solution bags. Be certain that the label specifically states that the medication is for IV use. IV fluid and electrolyte solutions come in a variety of volumes and concentrations in glass and plastic containers (see Box 12-1). • Gloves • Administration set with appropriate needle or needleless connector, drip chamber, and filter • Physiologic solution ordered • Syringe and needle or needleless connector (if giving by bolus) • Heparin (saline) lock adapter • Tape • Heparin (saline) solution, piggyback, and additional solutions as appropriate Additional supplies may be required to access, flush, or change IV administration sets, in-line filters, or dressings, depending on the type of peripheral, central, or implantable device being used. When selecting an IV site, consider the following: the length of time that the IV will be required; the condition and location of the veins; the purpose of the infusion (e.g., rehydration; delivery of nutritional needs [e.g., TPN], chemotherapy, and antibiotics); and the patient’s status, cooperation level, and preference for and amount of self-care needed for the injection site (if appropriate). Peripheral IV devices include the wing-tipped needle (see Figure 12-4), the over-the-needle catheter (see Figure 12-5, A), and the inside-the-needle catheter (see Figure 12-5, B). The over-the-needle catheters are the most commonly used venous access systems for entering the peripheral veins. If a prolonged course of treatment is anticipated, start the first IV in the hand (Figure 12-10). The metacarpal veins, the dorsal vein network, the cephalic vein, and the basilic vein are commonly used. To avoid irritation and leakage from a previous puncture site, the subsequent venipuncture sites should be made above the earlier site. Figure 12-11 shows the veins of the forearm area that could be used for additional venipuncture sites. Central IV access devices (see p. 173) are used for the following situations: when the purpose of therapy dictates (e.g., large-volume, high-concentration, or hypertonic solutions are to be infused); when peripheral sites have been exhausted as a result of repeated use or when the condition of the veins for access is poor; when long-term or home therapy is required; or when an emergency condition mandates adequate vascular access. The central veins that are most commonly used for central venous catheters are the subclavian and jugular veins. When upper body veins are not acceptable, the femoral veins may be accessed for short-term or emergency use. • Never recap, bend, or break used needles because of the danger of inadvertently puncturing the skin. • Use in-line filters as recommended by the manufacturer of the drug to be infused. • DO NOT mix any other drugs with blood or blood products (e.g., albumin). • DO NOT administer a drug in an IV solution if the compatibility is not known. • Drugs must be entirely infused through the IV line before adding a second medication to the IV line.
Parenteral Administration
Intravenous Route
Intravenous Therapy
![]() http://evolve.elsevier.com/Clayton
http://evolve.elsevier.com/Clayton
Objectives
Key Terms
 ) (p. 170)
) (p. 170)
 ) (p. 171)
) (p. 171)
 ) (p. 172)
) (p. 172)
 ) (p. 172)
) (p. 172)
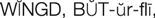
 ) (p. 172)
) (p. 172)
 ) (p. 173)
) (p. 173)
 ) (p. 173)
) (p. 173)
 ) (p. 173)
) (p. 173)
Equipment Used For Intravenous Therapy
Intravenous Administration Sets
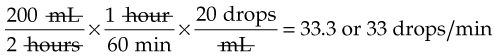
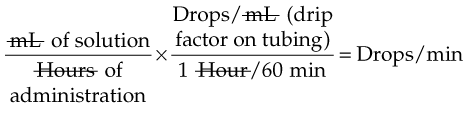
Equipment Used in Conjunction With Intravenous Therapy
Types of Infusion-Control Devices
Controllers
Pumps
Syringe Pumps
Intravenous Access Devices
Peripheral Access Devices

Central Access Devices
Intravenous Dose Forms
Objective
Key Terms
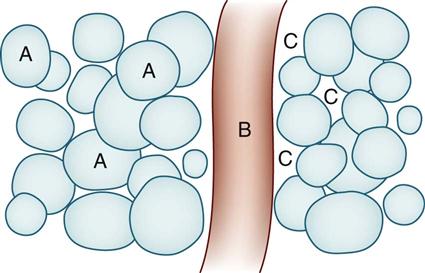
Types of Intravenous Solutions
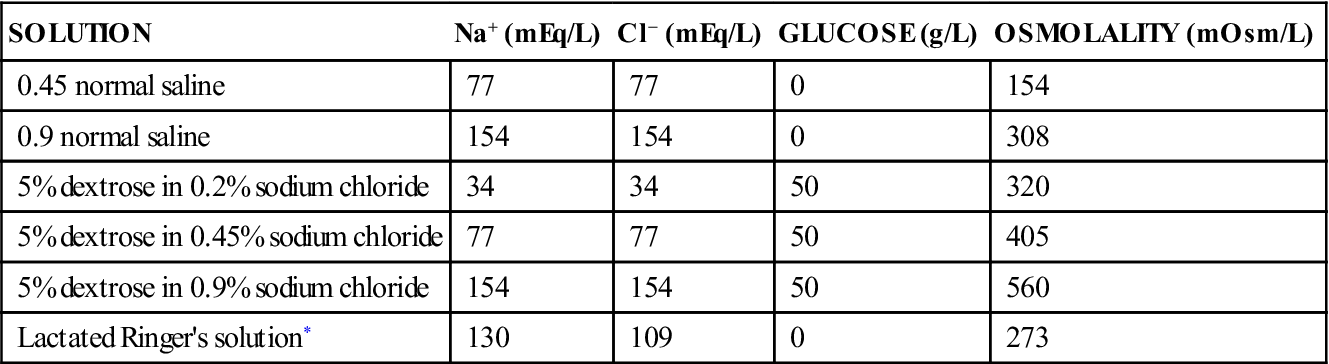
SOLUTION
Na+ (mEq/L)
Cl− (mEq/L)
GLUCOSE (g/L)
OSMOLALITY (mOsm/L)
0.45 normal saline
77
77
0
154
0.9 normal saline
154
154
0
308
5% dextrose in 0.2% sodium chloride
34
34
50
320
5% dextrose in 0.45% sodium chloride
77
77
50
405
5% dextrose in 0.9% sodium chloride
154
154
50
560
Lactated Ringer’s solution*
130
109
0
273
Large-Volume Solution Containers
Small-Volume Solution Containers
Administration of Medications by the Intravenous Route
Objectives
Key Term
Dose Forms
Equipment
Sites
Peripheral Intravenous Access
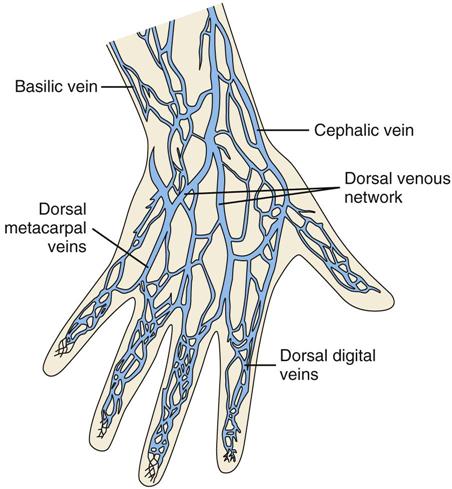
Central Intravenous Access
General Principles of Intravenous Medication Administration
12. Parenteral Administration: Intravenous Route
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


 ) (
) ( ) (
) ( ) (
) (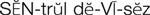 ) (
) (
 ) (
) ( ) (
) (
 ) (
) ( ) (
) ( ) (
) ( ) (
) (
 ) (
) (
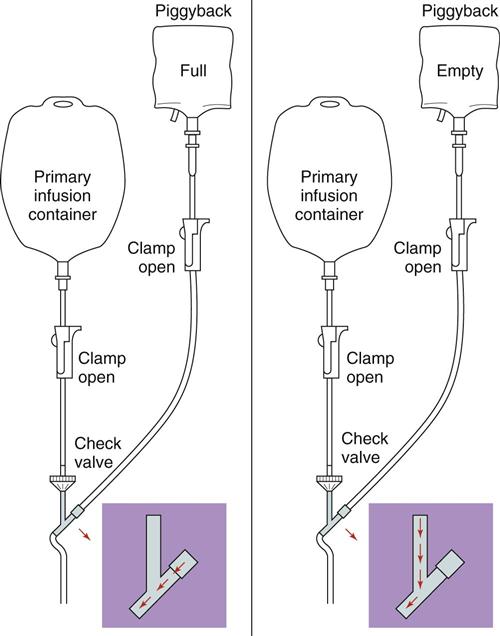
 ) (
) (