Dyspnea, a symptom, and airway obstruction, a sign, are most notably associated with primary lung cancer and metastatic disease (Torres-Carranza et al., 2006; Le 2005; Wickham, 2002; Chernecky & Shelton, 2001; Jones et al., 2001; Chernecky Sarna, 2000, Chernecky, 2004). In the clinical setting, dyspnea is commonly defined as shortness of breath, difficulty breathing, breathlessness, or a tight throat. It is a subjective symptom that often is not assessed, or considered of little relevance, and poorly managed.
The gold standard for assessment is patient self-reporting. In children, the parent or caregiver report, a change in the child’s level of activity, and changes in vital signs are the usual indicators. Dyspnea can be a problem for patients on ventilators (Bergbom-Engberg et al., 1989), and it also is common in pathological processes such as obesity, chronic obstructive pulmonary disease (COPD), anemia, anxiety, cachexia, and heart failure. Therapy-induced dyspnea can result from surgical thoracotomy, radiation-caused fibrosis of the lungs, and chemotherapy-related pulmonary and cardiac toxicities. Dyspnea in the cancer patient is usually multifactorial and includes symptom clusters (Esper & Heidrich, 2005), and affects the patient’s quality of life through decreasing physical and social functioning.
The two subcategories of dyspnea are dyspnea at rest and dyspnea with activity (also known as dyspnea on exertion). Once the type of dyspnea has been identified, it is quantified in time and/or relation to activity and impact on quality of life. Dyspnea is a predictor of an increased likelihood of hospital death rather than death at home. It is the one symptom that causes the greatest distress for caregivers. Dyspnea causes loss of physical stamina, therefore activities such as shopping, getting the mail, bathing, grooming, and dressing can become difficult, if not impossible. This change in activity status can lead to anger, helplessness, frustration, and depression. In particular, a fear of dying arises, because the patient feels like “I cannot breathe.” This feeling of drowning as a result of air hunger is real and should be discussed with the patient and caregiver at the appropriate time.
Airway obstruction is the occlusion of the airway passage that begins in the nose and ends in the lungs. Occlusions can be partial or complete. The diagnosis usually begins with the patient complaining of difficulty breathing, having something stuck in the throat, having a hard time breathing, or being winded. The result is a cycle of air hunger, lowering oxygen concentration, and anxiety. Interventions are started to break the cycle, and some interventions are provided immediately.
EPIDEMIOLOGY AND ETIOLOGY
The incidence of dyspnea is 21% to 90% in all cancer patients, with a predominance of 80% in patients with lung cancer. The incidence of airway obstruction is 30% (Chan et al., 2002) to 75% (Shohat et al., 2004). Dyspnea can take weeks to months to develop, and the onset often is gradual. Airway obstruction can be immediate or gradual in onset and result in either partial or complete obstruction. Both cancerous and noncancerous conditions can cause dyspnea and airway obstruction (Table 12-1 and Table 12-2).
| *Cancer treatment modalities also can cause airway obstruction. | |
| Cancerous Causes | Noncancerous Causes |
|---|---|
| Aortic tumor | Aberrant thymic tissue (infant) (Shah et al., 2001) |
| Head and neck cancer (esophagus, nares, oral cavity, pharynx, salivary gland, sinuses, tongue, tonsils, trachea) | AIDS |
| Hodgkin disease | Anaphylaxis |
| Lung | Cachexia |
| Lymphoma | COPD |
| Non-Hodgkin lymphoma | Cystic cervical thymus (infant) |
| Diaphragmatic excursion problem | |
| GERD | |
| Heart disease or failure | |
| Hypophosphatemia | |
| Hypersensitivity reaction | |
| Lung reduction surgery | |
| Neuromuscular abnormalities | |
| Obesity | |
| Pleural effusion | |
| Pneumonia | |
| Pneumothorax | |
| Pulmonary emboli | |
| Sarcoidosis | |
| Superior vena cava syndrome (SVCS) | |
| Thoracic scoliosis | |
| Thyroid toxicosis | |
| Tobacco use history | |
| Tuberculosis (TB) | |
| *Cancer treatment modalities also can cause airway obstruction. | |
| Cancerous Causes | Noncancerous Causes |
|---|---|
| Acute lymphoblastic leukemia: T cell with mediastinal mass (in child) (Howard et al., 2006) | Adenoids |
| B-cell lymphoma | Pseudotumor caused by motor vehicle air bag injury (Alaani et al., 2005) |
| Burkitt’s lymphoma (Choo et al., 2005) | Juvenile angiofibroma |
| Chordoma (in child) (Tao et al., 2005) | Juvenile hyaline fibromatosis |
| Head and neck cancer: adenoid cystic carcinoma, chondrosarcoma (nasal), craniopharyngioma, ectomesenchymoma (nose), esophageal, nares, olfactory neuroblastoma, oropharynx histiocytoma, salivary gland (Mehra & Woessner, 2005), sinuses, thyroid, tongue, tracheal paraganglioma | Narcotic administration, morphine (Byard & Gilbert, 2005) |
| Hodgkin disease | Papilloma (in child) |
| Lung | Papillomatous tissue during CO2 laser treatment |
| Meningioma (extracranial) | Postendotracheal tube/tracheostomy stricture |
| Multiple myeloma | Pseudotumor of lung |
| Non-Hodgkin lymphoma | Rheumatoid arthritis (Haben et al., 2005) |
| Ovarian (Tasci et al., 2005) | Superior vena cava syndrome (SVCS) |
| Plasmacytoma | Thyroid goiter |
| Renal metastasis (Torres-Carranza et al., 2006) | Tonsillitis |
| Schwannoma | Vocal cord paralysis with fixed stenosis |
RISK PROFILE
• Primary cancers of the lung, head, or neck (especially the esophagus), lymphoma, or metastasis to the lung.
• Superior vena cava syndrome (see Chapter 46), right-side heart failure, obesity, COPD, pneumonia, anemia, or anxiety.
• Radiation therapy to the lungs, endotracheal intubation (Chen et al., 2005), administration of narcotics (Byard & Gilbert, 2005), or laser ablation (Skoulas & Kountakis, 2003).
• History of tobacco use or exposure to asbestos (Dudgeon et al., 2001), radon (Carta et al., 2001), or Agent Orange (Pavuk et al., 2005).
• Pediatric population: Space-occupying lesions (e.g., metastatic tumors); tumor-associated effusions and infections (e.g., pneumonia or after an aspiration procedure); or severe skeletal or neuromuscular impairments that interfere with respiration (Kane & Himelstein, 2002). About 50% to 60% of all patients with T-cell acute lymphoblastic leukemia have a mediastinal mass; this is also a frequent finding in patients with Hodgkin’s or non-Hodgkin’s lymphoma (Howard et al., 2006).
PROGNOSIS
The prognosis depends on the cause of the dyspnea or airway obstruction and the effectiveness of treatment. For dyspnea, structured nursing programs are effective for patients with lung cancer (Moore et al., 2002; Bredin et al., 1999; Sarna, 1998; McCorkle et al., 1989). These interventions have not been tested in the pediatric population. For airway obstruction, effective treatments include radiation therapy, surgery, endoscopic laser ablation (Venuta et al., 2002), photodynamic therapy (Jones et al., 2001), and stenting (Chan et al., 2002). With metastatic disease, only palliative treatment is possible.
PROFESSIONAL ASSESSMENT CRITERIA (PAC)
1. Inspect and assess for cyanosis, tachypnea, stridor (See & Olopade, 2005), dyspnea (Ayers & Lappin, 2004), shortness of breath, use of accessory muscles, and capillary refill longer than 3 seconds.
2. Assess for hypoxemia: PaO2 less than 92% and/or abnormal ABGs; unexplained respiratory alkalosis may be an early sign of sepsis and can progress to metabolic acidosis (Lynch, 2006).
3. Measure dyspnea on a 0 to 10 scale, with 0 being no dyspnea and 10 being severe dyspnea. Measures should be compared from one assessment to the next assessment. Note whether dyspnea occurs at rest and with exertion. The Visual Analogue Scale (Corner et al., 1995) and the numeric rating scale (Gift & Narsavage, 1998) have been validated for use in adults, and the Dalhousie Dyspnea Scale has been validated for use in children age 8 or older (McGrath et al., 2005). Consider using terms and phrases such as shortness of breath, tight throat, difficulty breathing, breathlessness, and labored breathing to enhance the patient’s understanding of dyspnea.
4. Assess vital signs for hypotension, dyspnea, tachycardia, tachypnea, stridor, chest pain, and fever (for pulmonary infection).
5. Auscultate the lung fields for crackles, adventitious breath sounds, and bilateral inequality; visually assess for decreased or unequal chest expansion and decreased diaphragmatic excursion.
6. Observe for anxiety, restlessness, and change in level of consciousness. Asking the patient to rate his or her anxiety on a 0 to 10 scale (0 meaning “no anxiety” and 10 meaning “the worst anxiety I could ever have”) may be useful for assessing anxiety and its severity.
7. Obtain and assess a chest x-ray film (CXR) for obstruction (Fig. 12.1, A), pleural effusion, or other abnormalities (Chernecky & Berger, 2004). The lateral view (Fig. 12.1, B) can allow a gross estimate of the degree of airway compromise by a mediastinal mass (Guillerman et al., 2005).
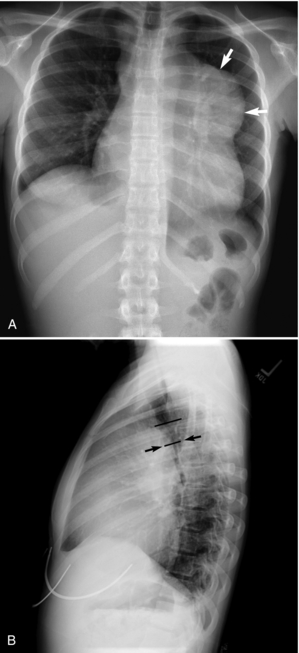 |
| Fig. 12.1A 15-year-old girl diagnosed with Hodgkin’s lymphoma. A, Anteroposterior chest x-ray film shows a large mediastinal mass (arrows). B, Lateral chest x-ray film shows compression of the distal trachea (arrows) by the mediastinal mass. From Guillerman, R. P., Braverman, R. M., & Parker, B. R. (2006). Imaging studies in the diagnosis and management of pediatric malignancies. In P. A. Pizzo & P. G. Poplack. Principles and practice of pediatric oncology. (5th ed.). Philadelphia: Lippinocott Williams & Wilkins. |
8. Obtain and assess a computed tomography (CT) scan and/or endobronchial ultrasound (EBUS) scan (Chernecky & Berger, 2004). CT (Fig. 12.2) is considered the most accurate imaging modality for detecting airway compromise (Guillerman, 2005).
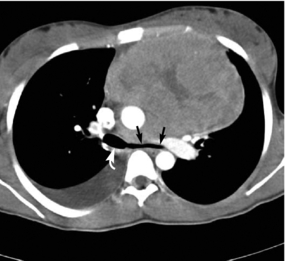 |
| Fig. 12.2The same patient as in Fig. 12.1, A. The CT image shows marked compression of the left mainstem bronchus (straight arrow) compared to the normal-caliber right mainstem bronchus (curved white arrow). From Guillerman, R. P., Braverman, R. M., & Parker, B. R. (2006). Imaging studies in the diagnosis and management of pediatric malignancies. In P. A. Pizzo & P. G. Poplack. Principles and practice of pediatric oncology. (5th ed.). Philadelphia: Lippinocott Williams & Wilkins. |
9. Obtain and assess laboratory test and biopsy results (Chernecky & Berger, 2004): WBC total and differential to assess for neutropenia; RBCs, hematocrit, and hemoglobin to assess for anemia (Lynch, 2006); electrolytes; lung or other biopsy.
10. Obtain pulmonary function tests and assess for restrictive or obstructive disease. In patients with cancer, this is indicated by decreases in:
• Total lung capacity (TLC)
• Diffusion capacity (DLCO)
• Forced vital capacity (FVC)
• Forced expiratory volume in 1 second (FEV1)
• FEV1/FVC%
• Residual volume (RV)
• Peak expiratory flow (PEF)
• Forced expiratory reserve (FER)
11. Obtain sputum for culture and sensitivity.
12. Assess for right-side heart failure/cor pulmonale: ECG changes (Chernecky et al., 2006), CVP changes, dependent peripheral edema, fatigue, nausea, anorexia, weight gain, hepatomegaly, jugular vein distention (JVD), positive hepatojugular (HJ) reflex, systolic or diastolic murmur, prominent S2, polyuria at night, and ascites.
NURSING CARE AND TREATMENT
1. For airway obstruction, consider preparing the patient for high-dose brachytherapy (Allison et al., 2004), chemotherapy, surgical resection, stenting (Allison et al., 2004), tracheotomy, or intubation; or, in the case of lung cancer, for YAG laser therapy (Venuta et al., 2002). Also prepare the patient for diagnostic tests (e.g., EBUS or CT scanning of the chest) (Herth et al., 2003).
2. For dyspnea, prepare the patient for use of medications (steroids, morphine, bronchodilators, anxiolytic agents), inhalation therapy (metered-dose inhaler, nebulized morphine, furosemide, or fentanyl citrate or IPPB), and oxygen therapy (Chernecky, 2005). Theophyllines have not been shown to be effective in treating dyspnea in patients with lung cancer, as they have in patients with COPD.
3. For patients with non-small cell lung cancer, prepare the patient for photodynamic therapy (PDT) to help relieve airway obstruction and dyspnea (Jones et al., 2001).
4. Elevate the head of the bed to high Fowler’s position to relieve airway obstruction and dyspnea in patients with tumors that are not interthoracic. For patients with interthoracic tumors, placing the good lung down may increase ventilation. The nurse and patient may need to evaluate various patient positions to maximize air exchange and facilitate mobilization of secretions.
5. Obtain and interpret O2 saturation values (signs of hypoxia include restlessness, dyspnea, anxiety, and cyanosis).
6. Administer oxygen to treat hypoxia and high-dose corticosteroids (over 100 mg/day or 1 mg/kg) for inflammation. The use of air blowing through the nose has been shown to be effective in reducing dyspnea (Philip et al., 2006).
7. In adults, maintain venous access via a peripheral IV line with an 18-gauge needle or use a venous access device (VAD). For children, use a peripheral venous device that is age appropriate in size.
8. Administer low-dose morphine sulfate and/or an anxiolytic agent for anxiety and dyspnea.
9. Encourage the use of diaphragmatic breathing or pursed-lip breathing, which uses the lips as a mild resistor to prolong exhalation. This increases airway pressure, delaying compression of the airway and minimizing air trapping.
10. Activity: bed rest with bathroom privileges as tolerated; may gradually increase activity, with planned rest periods, as dyspnea resolves.
11. Use relaxation techniques such as imagery or music therapy to reduce anxiety (Gift et al., 1992).
12. Consider whether uncontrolled coughing that leads to dyspnea may be controlled with cough suppressants (dextromethorphan or opioids).
14. Consider the use of antibiotic therapy to treat bacterial pneumonia.
15. Provide referral to clinical nurse specialist, home health nurse, hospice, social worker, and/or respiratory therapist as appropriate.
EVIDENCE-BASED PRACTICE UPDATES
Review of the literature reveals an impressive lack of recent evidence-based reports. In many studies, the term breathlessness is used instead of dyspnea (Froggatt & Walford, 2005; Johnson & Moore, 2003; Hoyal et al., 2002; Krishnasamy et al., 2001; Bredin et al., 1999; Connolly & O’Neill, 1999; Corner et al., 1996). Breathlessness is presented as a symptom commonly seen in advanced cancer and is described as the subjective sensation of an uncomfortable awareness of breathing or difficulty breathing (Corner et al., 1996). It is seen as a complex symptom involving physical, psychological, emotional, and functional factors (O’Driscoll et al., 1999).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


