- Liver and biliary tract: anatomy, physiology and function
- Bilirubin metabolism and excretion
- Albumin – a marker of liver synthesis
- Liver enzymes (ALT, GGT and AP): markers of liver injury
- Laboratory test results in liver and biliary tract disease
- Adult jaundice
- Neonatal jaundice
This chapter is concerned with the measurement of the concentration of five substances in blood plasma/serum. Although structurally and functionally distinct they are treated together here because they are routinely measured together in a profile commonly known as liver function tests (LFTs). The principle use of the profile is identification of patients who are suffering liver or biliary tract disease. Depending on the exact nature of liver disease, one or more of these tests may be normal but it is unlikely that all would be normal in any patient suffering significant liver or biliary tract disease. Thus, the combination of five LFTs has more power to detect liver or biliary tract disease than each individual test. None of the five tests are specific for a particular liver or biliary tract disease, or indeed specific for liver/biliary tract disease generally, so that there are other diseases, not involving the liver or biliary tract, in which one or more of these tests might be abnormal.
Normal anatomy and physiology
The liver
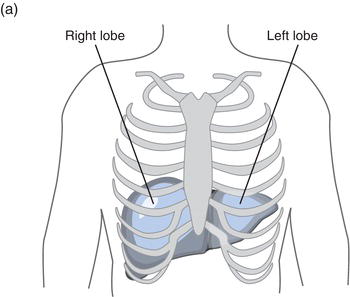
The liver, the largest internal body organ weighing around 2 kg, is located in the right upper quadrant of the abdomen, protected for the most part by the lower rib cage. (Figure 11.1a) The narrower left lobe extends from the rib cage over the stomach. It is red-brown in colour due to its copious blood supply: the organ receives around 30% of total cardiac output every minute from two sources, the portal vein and hepatic artery. The products of ingested food are transported to the liver directly from the gastrointestinal tract in blood via the portal vein and oxygenated blood is supplied via the hepatic artery. Blood leaves the liver by way of the hepatic vein, draining to the inferior vena cava for return to the heart (Figure 11.1b).
The complexities of liver function can be broadly summarised under three interconnected headings:
- Metabolic function.
- Synthetic function.
- Excretory function.
Hepatocytes, the cells the bulk of the liver is composed of, play a central role in the metabolism of ingested carbohydrates, proteins and fats. That is why these products of digestion are transported first to the liver, via the portal vein. Glucose derived from ingested carbohydrates can be stored until required, in hepatocytes, as glycogen. Between meals when glucose is in short supply, these glycogen stores are mobilised. During starvation, when even glycogen stores are depleted, hepatocytes are able to convert amino acids derived from ingested and body proteins to glucose. By these effects on carbohydrate metabolism, the liver plays a central role in regulating blood glucose concentration.
Dietary derived amino acids are synthesised into proteins in the liver; these include many of the proteins, like albumin, that are present in blood plasma. The vital process of blood clotting depends on the liver synthesis of the specific plasma proteins (clotting factors) of the clotting cascade. Urea, a waste product of amino acid metabolism is synthesised in the liver before transport in blood to the kidneys, where it is excreted in urine. The liver plays a major role in the metabolism of ingested fats (lipids) by synthesising the lipoproteins necessary for transport of these fats, including cholesterol and triglycerides, around the body in blood. Synthesis of bile acids from cholesterol in the liver provides a further example of the way liver affects lipid metabolism and leads nicely to the excretory role of the liver.
Production of bile – the biliary tract
The liver is the source of bile, an alkaline watery yellow/green fluid that contains the bile acids produced from cholesterol. These bile acids have to be conveyed in bile to the intestine where they are required for absorption of dietary lipids. Bile also provides the route for excretion of drugs and other waste products of metabolism that are not excreted from the body by kidneys in urine. The production of bile thus fulfils the excretory role of the liver.
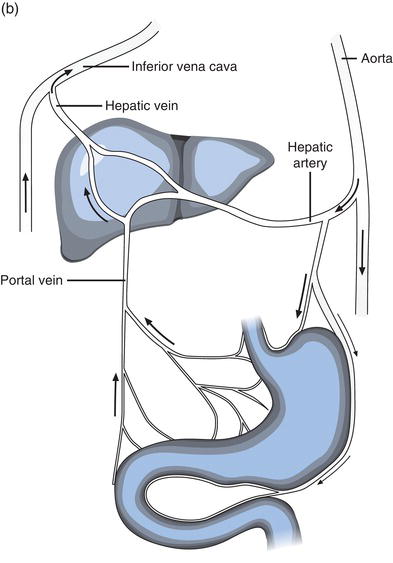
Bile is produced within and secreted from hepatocytes to microscopic tube like structures called bile canaliculi that coalesce within the liver to form larger bile ducts. These in turn join to form the larger hepatic bile ducts that conduct bile from the liver. Left and right hepatic bile ducts join outside the liver forming the common hepatic bile duct, and this is connected via the common bile duct to the duodenum of the intestinal tract. The pancreatic duct that conveys pancreatic digestive juice from the pancreas joins the common bile duct a few centimetres from the duodenum, so that bile and pancreatic juice enter the intestinal tract through the same opening in the duodenum. Bile production by the liver is continuous, amounting to around 500 ml/day. Between meals, when its digestive property is not required, the passage of bile (and pancreatic juice) to the duodenum is blocked by closure of a sphincter (sphincter of Oddi) sited at the junction of common bile duct and duodenum. When this sphincter is closed bile is stored in the gall bladder, which is connected to the common bile duct via the cystic duct. When food is ingested the sphincter opens and bile passes from the gall bladder via cystic duct and common bile duct, to the lumen of duodenum. The biliary tract is the totality of structure that conveys bile from hepatocytes to the intestine and includes bile ducts (cananliculi) within the liver, hepatic bile ducts, gall bladder, common bile duct and sphincter of Oddi.
Although bile acids represent a major constituent of bile, it is by no means the only one; bile is a chemically complex fluid containing of course water but also electrolytes and many waste products of metabolism destined for excretion in faeces. Among them is bilirubin, a waste product of haemoglobin catabolism.
Bilirubin
Many patients suffering liver or biliary tract disease have a yellow discoloration of the skin and mucous membranes, first evident in the sclerae (whites) of the eye. This clinical sign, known as jaundice (derived from the French word for yellow, jaune) which, as will become clear, is not confined to those suffering liver or biliary tract disease, is due to an abnormally high concentration in the blood of the potentially toxic yellow pigment, bilirubin.
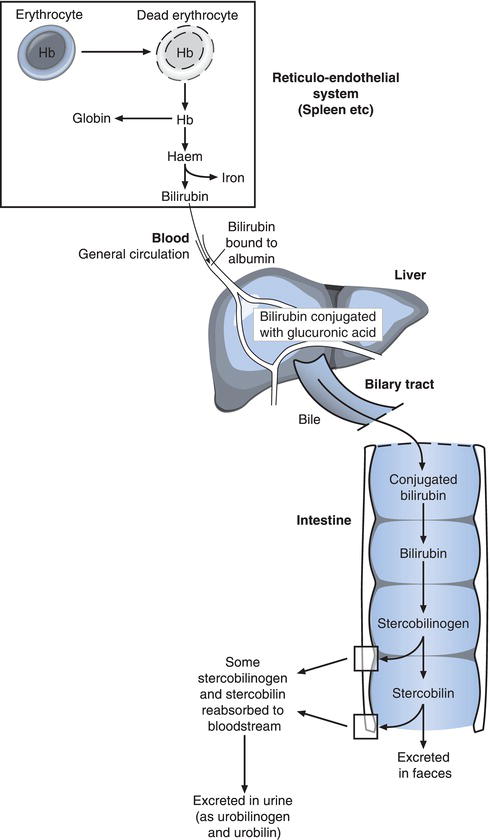
Most of the 250–300 mg bilirubin normally produced each day is derived from the breakdown (catabolism) of haemoglobin, the oxygen carrying protein contained in red blood cells (Figure 11.2). At the end of their normal 120 day life span, red blood cells are removed from circulating blood to the spleen and other parts of the reticuloendothelial (RE) system. In macrophage cells within the RE system, haemoglobin released from dead red cells is split into its constituent parts: haem and globin. The haem portion is converted to bilirubin, which is then tightly bound to albumin for transport in blood from RE-macrophages to the liver and eventual excretion in bile. Before entry to liver cells bilirubin is released from albumin.
Bilirubin is water insoluble and must be made water soluble for excretion in bile. This is achieved within hepatocytes by conjugation (joining) of bilirubin to a substance called glucuronic acid. The resulting so called conjugated bilirubin is secreted from the hepatocyte to bile canaliculi and onwards in bile, out of the liver via the biliary tract to the duodenum. The action of bacteria normally present within the gut deconjugates bilirubin (that is releases bilirubin from glucuronic acid) and converts bilirubin to stercobilinogen and then stercobilin. Almost all of these final products of bilirubin metabolism are excreted from the body in faeces (stercobilin is an orange-brown pigment responsible for the brown colour of faeces). However a little stercobilinogen and stercobilin is reabsorbed to blood during passage through the gut. Most of this is recycled by the liver and re-excreted into the gut, but some is excreted in urine as urobilinogen/urobilin (urobilinogen/urobilin are the names given to stercobilinogen/stercobilin when they are not in the intestine or faeces).
The bilirubin that is normally present in blood plasma/serum and measured in the laboratory is comprised of two fractions: unconjugated bilirubin and conjugated bilirubin. In health by far the biggest proportion is unconjugated bilirubin; this is the bilirubin that is being transported (bound to albumin) in blood to the liver for excretion. Conjugated bilirubin is, as outlined already, the form in which bilirubin is excreted from the liver in bile to the intestine. Now, although nearly all conjugated bilirubin is normally excreted via bile and gastrointestinal tract in faeces, a very small proportion returns to blood, from hepatocytes, bile canaliculi or intestine. The bilirubin test routinely performed in the laboratory measures total bilirubin concentration (i.e. the sum of unconjugated bilirubin – normally around 80–90% of total bilirubin and conjugated bilirubin normally 10–20% of total bilirubin). The distinction between unconjugated and conjugated bilirubin is important in the differential diagnosis of the jaundiced patient because depending on its cause, jaundice can result from either predominant increase in unconjugated bilirubin or predominant increase in conjugated bilirubin. Most laboratories have the capacity to measure not only total bilirubin concentration but also conjugated bilirubin concentration, in the relatively rare circumstance that this extra testing is diagnostically necessary.
Albumin
Blood plasma contains a mixture of many proteins each with its own function. These include proteins required to fight infection (immunoglobulins or antibodies), enzymes, blood clotting factors, specific transport proteins and many more. Albumin is the single most abundant protein in plasma comprising as it does around 40–60% of total plasma protein; it is the most frequently measured plasma protein in clinical laboratories.
Like many other proteins present in plasma, albumin is synthesised from amino acids in the liver. It has two main functions. The first of these is as a transport protein. Many water insoluble substances can only be transported in blood plasma when bound to specific proteins. Albumin is one of these proteins and is, as we have already seen, required for transport of unconjugated bilirubin. Around half the calcium present in blood plasma is bound to albumin, as are free fatty acids and many drugs.
The other important function of albumin is maintenance of blood plasma volume. As the single most abundant protein in plasma, albumin is a major contributor to colloid osmotic pressure or plasma oncotic pressure. This pressure opposes the tendency of fluid to escape from capillary blood vessels into the surrounding interstitial space due to blood pressure within vessels. Oedema (which may be visible as swelling) is the term used to describe an abnormal accumulation of fluid in the interstitial space and this occurs if albumin concentration of plasma and therefore plasma oncotic pressure fall below normal.
Solution of albumin may be administered therapeutically via an intravenous line to patients who have suffered major trauma, burns or clinical shock, to restore plasma volume.
Gamma glutamyl transpeptidase (GGT), alanine transferase (ALT) and alkaline phosphatase (AP)
GGT, ALT and AP are all enzymes. They are present in the cells of the liver and biliary tract where they function as catalysts of specific metabolic reactions that are required for cell function and survival. The enzymes have no function in blood plasma and the small amount that is normally present (compared with that in cells) is due to normal cell turnover. As cells naturally die they release their contents to blood plasma. The cell injury or death (necrosis) associated with liver or biliary tract disease results in increased amounts of these intracellular enzymes being released to blood plasma. These enzyme measuring tests then are not strictly speaking measuring liver function, they are merely convenient blood markers of liver/biliary tract cell injury/death.
If the source of these enzymes were only the cells of liver or biliary tract tissue, then raised levels would always indicate liver or biliary tract disease. In fact, although GGT, ALT and AP are frequently referred to as ‘liver enzymes’, they are also present in other tissues, and damage or disease of these non-liver, non-biliary tract tissues may also be associated with a rise in serum concentration of either GGT, ALT or AP. The most significant non-liver non-biliary tract source of GGT is the pancreas. ALT is present predominantly in the hepatocytes of the liver but also, albeit at far lower concentration, in kidney tissue, heart muscle (myocardium) and skeletal muscle. AP is present not only in the liver cells of the bile canaliculi, but also in bone cells, intestinal tissue cells and placental tissue cells.
Laboratory measurement of liver function tests (LFTs)
Patient preparation
No particular patient preparation is necessary.
Timing of sample
Blood for LFTs may be sampled at any time. It is however important that there is not undue delay (more than a few hours) in transporting specimens to the laboratory for separation of serum (or plasma) from cells.
Sample requirement
Around 5 ml of venous blood is required for LFTs. Blood should be collected into a plain tube without additives if local policy is to use serum for analysis, and into a tube containing the anticoagulant lithium heparin if local policy is to use plasma. A falsely raised albumin level occurs if a tourniquet is left in position for a more than a minute or two before sampling blood. If possible the use of a tourniquet should be avoided. Bilirubin is slowly destroyed by both artificial and sun light, leading to falsely low results. To reduce this effect samples should be protected as far as possible from exposure to light both before and during transport to the laboratory.
Neonatal measurement of bilirubin only
For reasons still to be discussed new born babies (particularly those born prematurely) frequently need monitoring of serum bilirubin concentration. In these cases only bilirubin need be measured, reducing the sample requirement to 0.5 ml or less. Capillary blood obtained by a heel stab (Chapter 2) is usually used in these circumstances. Particular care is required to avoid haemolysis during blood collection as such samples are unsuitable for bilirubin estimation. A special dark plastic container (to protect bilirubin from exposure to light) is often used for collection of blood samples for neonatal bilirubin measurement.
Interpretation of results
Approximate reference ranges
Plasma/serum bilirubin (total) | <21 µmol/L |
Plasma/serum albumin | 35–50 g/L |
Plasma/serum ALT, GGT and AP. Methods used to measure enzyme levels vary between laboratories; each method has its own reference range. It would be misleading therefore to quote even approximate reference ranges for these enzyme tests. Always use local laboratory reference range when interpreting results. The unit of measurement for all enzyme assays is the international unit (IU) and results are expressed in terms of number of IUs per litre of plasma/serum (i.e. IU/L).
Bone is a source of AP, and during periods of bone growth (childhood and adolescence) the amount of AP in plasma/serum is markedly increased compared with that which prevails in adulthood. Each laboratory publishes AP reference ranges that refer to specific age ranges. It is vitally important the age of the patient be taken into account when interpreting AP results. The presence of AP in placental tissue determines that AP is higher during pregnancy.
Causes of raised bilirubin – jaundice
The concentration of bilirubin in serum reflects the balance between the amount produced by the normal process of red cell destruction and that removed from the blood by the liver and excreted in bile. An abnormally raised serum bilirubin occurs in three broad pathological situations:
- Diseases in which the rate of bilirubin production exceeds the normal rate of liver processing and excretion. Bilirubin consequently accumulates in blood. These are the haemolytic anaemias, which are characterised by increased red cell destruction and therefore increased catabolism of haemoglobin and consequent increased bilirubin production. Liver and biliary tract are functionally normally, so that haemolytic anaemias serve to remind that jaundice can occur in patients without liver or biliary tract disease. The jaundice that occurs in these conditions (called haemolytic jaundice) is the result of increase in unconjugated (rather than conjugated) bilirubin.
- Diseases in which rate of bilirubin production is normal but the rate at which the liver can process a normal bilirubin load is compromised by disease. These are diseases solely of the liver and the resulting jaundice (called hepatocellular jaundice) is the result of predominant increase in conjugated (rather than unconjugated) bilirubin.
- Diseases in which the rate of production of bilirubin is normal, the rate at which liver cells process bilirubin is normal but the rate of excretion is reduced due to disease of the biliary tract (either within the liver or at some other point in the biliary tract between the liver and the duodenum). The disorders in this group are characterised by cholestasis, which means reduction in bile flow. The resulting jaundice (called cholestatic or obstructive jaundice) is caused by a predominant increase in conjugated (rather than unconjugated) bilirubin.
Generally speaking haemolytic jaundice is associated with only a slight increase in bilirubin; concentration rarely rises above 70 µmol/L. An important exception is haemolytic disease of the newborn (discussed further). Since the liver is functionally normal in this condition, all other LFTs are normal.
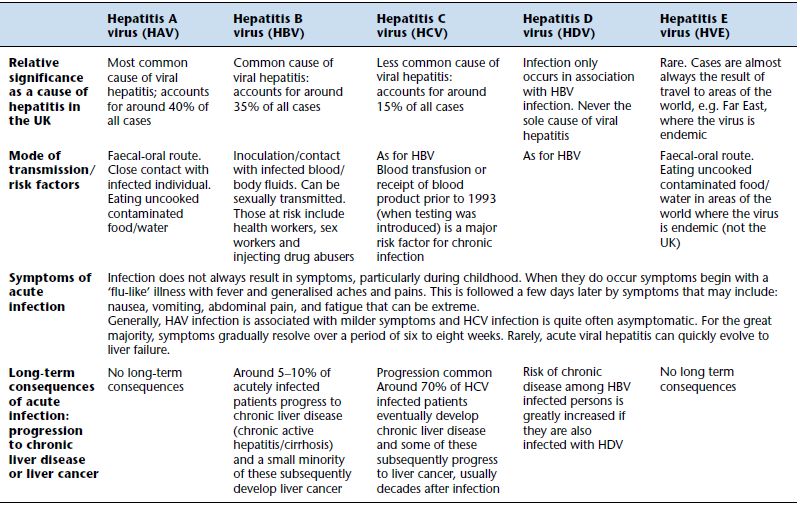
Higher serum bilirubin concentration is seen in liver disease in which there is injury to liver cells. For example, acute inflammation of the liver (acute hepatitis), which is usually the result of viral infection (Table 11.1) or alcohol abuse (acute alcoholic hepatitis), causes peak concentrations (up to 300 µmol/L) at around the tenth day after symptoms develop. During the four to eight week recovery period, bilirubin gradually returns to normal. In some cases inflammation does not resolve and becomes chronic and progressively destructive. The term chronic active hepatitis is used to describe such cases, and bilirubin, though not as high as during acute hepatitis, may remain raised. Cirrhosis, that is irreversible liver damage, most often the result of prolonged alcohol abuse or unresolved viral hepatitis (there are many rarer causes), may be associated with a near normal serum bilirubin concentration in the early stages but rises as the disease progresses, often over many years, to liver failure. Secondary spread (metastases) of primary cancers to the liver is often associated with raised bilirubin; jaundice is a poor prognostic sign in patients with such secondary cancer spread.
Table 11.1 Viral causes of hepatitis and therefore abnormal liver function tests.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree