Section Eleven Blood Product Administration
PROCEDURE 72 Administration of Blood Products
PROCEDURE 74 Massive Transfusion
PROCEDURE 75 Blood and Fluid Warmers
PROCEDURE 76 Blood and Fluid Pressure Infusers
PROCEDURE 77 General Principles of Autotransfusion
PROCEDURE 78 Autotransfusion Devices: Pleur-Evac
PROCEDURE 79 Autotransfusion Devices: Argyle
PROCEDURE 72 Administration of Blood Products
This procedure contains information on administering red blood cells (RBCs), whole blood, platelets, plasma, and cryoprecipitate.
See Procedures 73 to 76 for information regarding blood filters, massive transfusion, fluid warmers, and blood and fluid pressure infusers.
GENERAL PRINCIPLES (Rosenthal, 2004; Simmons, 2003; Simpson, 2006)
1. The intended recipient must be positively identified before a transfusion is started. This is the single most important step in preventing transfusion reactions.
2. Risks versus benefits need to be explained to the patient. Informed consent is required before a transfusion unless the patient’s condition is emergent.
3. A filter designed to retain blood clots and particles must be used in the transfusion.
4. Verify patency of the patient’s intravenous (IV) line (20- to 18-G or larger is preferred; however, RBCs can be administered through 22- to 25-G catheters).
5. Components should be mixed thoroughly before administration.
6. No medications or solutions should be added to, or transfused concurrently with, blood components except normal saline solution.
7. Lactated Ringer’s solution or other electrolyte solutions containing calcium should never be administered concurrently with a blood component mixed with an anticoagulant containing citrate, because calcium binds to citrate.
8. If the integrity of any component is questioned on visual inspection, it should be returned to the blood bank for further evaluation.
9. If the blood component container is entered for any reason, the component expires after 4 hours at room temperature (20° to 24° C) or after 24 hours if refrigerated at 1° to 6° C (AABB, 2002).
10. All blood or blood components that are not used within 30 minutes must be stored in a monitored refrigerator that has been approved by the blood bank.
11. Blood components can be warmed if clinically indicated during massive transfusion or exchange; warming should be done through the use of an FDA-approved device that will not cause hemolysis.
12. Transfusion reactions can be life threatening and occur with exposure to even a small amount of blood; therefore, transfusions should be started slowly and vital signs should be obtained no more than 15 minutes after the transfusion is started.
13. Transfusions should be completed within 4 hours and before the expiration date/time of the blood component (AABB, 2002).
PATIENT PREPARATION
1. Obtain informed consent per the policy of the institution. Most policies waive consent in emergency situations but consider that the patient’s religious beliefs may prohibit the administration of blood or blood products.
2. Obtain specimens for type and crossmatch as required.
3. Establish vascular access, preferably a 20-G or larger catheter.
4. Prime Y-type blood tubing with normal saline, which is indicated for most blood products. Ensure the filter is covered with blood.
5. Assess and document vital signs, including blood pressure, heart rate, respiratory rate, and temperature.
RED BLOOD CELLS
RBCs, or packed red blood cells (PRBCs), are prepared by removing approximately 250 ml of the plasma from whole blood; therefore, there are no significant amounts of clotting factors or platelets in RBCs. Each unit of RBCs contains 250 to 300 ml. One unit of RBCs can increase an average adult’s hemoglobin by 1 g/dl, and hematocrit can increase by up to 2% to 3% (Rosenthal, 2004).
Indication
To increase the oxygen-carrying capacity of the circulatory system in the presence of acute or chronic blood loss (American Red Cross, 2003a).
Contraindications and Cautions
1. Removal of the plasma reduces the chance of adverse reactions from RBCs that might occur with the use of whole blood; however, transfusion reactions may still occur (Simpson, 2006).
2. Always have a second person check typing, crossmatching, expiration date, blood unit number versus unit number on the transfusion slip, and client identification. Document these data according to the hospital’s policy. Most major or fatal transfusion reactions result from type mismatches caused by a clerical error, administration of blood to the wrong patient, or incorrect identification of the blood component (Beyea & Majewski, 2003). In emergency situations, O-negative blood can be administered until crossmatched blood is available; O-positive blood may be used in postmenopausal females and males of any age.
3. Monitor fluid balance carefully in patients at risk for fluid overload.
4. Do not allow the RBCs to stand at room temperature longer than 30 minutes before administration (Simpson, 2006).
5. Infusion time should not exceed 4 hours. The longer the RBCs are left at room temperature, the greater is the danger of bacterial proliferation and RBC hemolysis (Simpson, 2006).
Procedural Steps
1. Check the expiration date on the blood.
2. Identify the patient according to institutional policy. With another person, compare the information on the blood record with the patient’s identification bracelet.
3. Invert the RBCs gently several times to achieve suspension.
4. Close roller clamp on tubing.
5. Spike one tail of Y-type tubing with 0.9% normal saline solution, and prime the tubing.
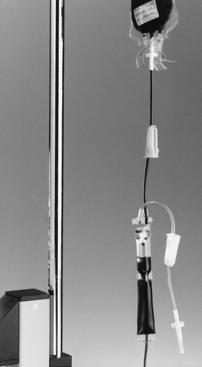
6. Spike the second tail of the Y-type tubing with the RBC bag. Ensure the drip chamber is half full and the filter is covered with saline to prevent damage to the blood cells (Figure 72-1).
7. After cleaning the IV port with an alcohol swab, attach the Y tubing to the patient’s IV site and open the roller clamp on the RBCs. A gentle squeeze on the filter helps start the flow of blood.
8. Infuse slowly for the first 15 minutes while observing the patient for reactions. Most serious reactions occur during this time. This step assumes that the patient is not in need of multiple, rapid, life-sustaining transfusions.
9. After 15 minutes, reassess the vital signs and adjust the flow rate to the desired speed if no signs of a transfusion reaction are noted.
10. Continue the assessments of the patient (always include the vital signs) throughout the transfusion as needed, according to the patient’s condition, and the number of units being administered.
11. After the infusion is complete, reassess the vital signs and flush the tubing with normal saline solution. If the transfusion therapy is complete, either discontinue the IV or hang the prescribed solution with new IV tubing.
12. Continue to monitor vital signs for at least 1 hour post transfusion (Simpson, 2006).
Age-Specific Considerations (Simmons, 2003)
1. Fluid overload is of concern in both elderly and young patients. Assess lung sounds before and at the completion of the transfusion.
2. There is a risk of viral infection during a transfusion. Cytomegalovirus (CMV) infection can be found on white blood cells. Those at risk for CMV infection are premature infants and immunocompromised patients.
WHOLE BLOOD
Whole blood may be either stored or fresh. One unit of whole blood contains approximately 450 to 500 ml. Whole blood is rarely used in any hospital setting as blood components specific to the patient’s needs, such as clotting factors, platelets, plasma, and RBCs, are used instead. Currently, the main use of whole blood is for massive transfusion (Rosenthal, 2004).
ALBUMIN
Albumin is a circulating protein found in both the serum and the extravascular area. The primary function of albumin is the maintenance of normal colloid oncotic pressure. Albumin solutions are available in 5% and 25% solution concentrations (Baxter Healthcare Corporation, 2003).
Indications (Baxter Healthcare Corporation, 2003)
1. To replace volume after an acute loss, a therapeutic phlebotomy, or a plasma exchange.
2. To correct hypoalbuminemia.
3. May be used to improve intravascular volume in patients who have severe burns or who are developing signs of edema.
4. May be used in conjunction with a diruetic in patients with pulmonary edema or adult respiratory distress syndrome.
Procedural Steps (Baxter Healthcare Corporation, 2003)
1. Check that the proper solution is being used (normal saline solution or D5W).
2. Spike the bottle with a vented spike, close roller clamp on the tubing, and attach IV tubing to the vented spike opening.
3. Squeeze the drip chamber until the chamber is one-third full.
4. Open the regulating clamp and prime the IV tubing.
5. Attach tubing to the patient’s IV access port after cleaning with an alcohol swab.
6. In patients experiencing hypovolemic shock, administer albumin at 1 to 2 ml/min. In other patients, administer at 2 to 4 ml/min for 5% albumin solutions and no faster than 1 ml/min for 25% albumin solutions.
7. Use of filters during albumin administration is determined by the manufacturer and institutional policy.
PLATELETS
Platelets play an important role in blood coagulation and thrombus formation. One unit of platelets can increase an average adult’s platelet count by 5000 platelets per microliter (Rosenthal, 2004). Platelets are obtained through centrifugation of whole blood (Simpson, 2006).
Contraindications and Cautions
1. Platelets must be infused within 4 hours of their expiration time and date (Rosenthal, 2004).
2. Transfusion reactions are possible owing to the plasma in which the platelets are stored (Rosenthal, 2004).
3. Platelets must be transfused through a 170- to 220-micron filter. Do not use filters that were previously used to filter whole blood or PRBCs (Rosenthal, 2004).
4. Most adults do not require ABO crossmatching before platelet infusion, unless they have had multiple platelet infusions and have become resistant to pooled donor platelets (Rosenthal, 2004).
5. Infants and small children do require ABO crossmatching or reduced-volume ABO incompatible platelets (Rosenthal, 2004).
6. Contraindicated in heparin-induced thrombocytopenia (HIT) (Simpson, 2006).
Procedural Steps
1. Prime the blood administration set with a normal saline solution.
2. Check the platelet blood type versus the patient’s blood type. It is not necessary to have the same ABO type, but it is preferred (American Red Cross, 2003b). Before exposure to Rh-positive platelets, anti–Rh-immune globulin should be administered to Rh-negative women who are of childbearing age (American Red Cross, 2003c).
3. Hang the platelet bag on one tail of the Y set.
4. Close the line roller clamp on the normal saline solution, and open the platelet line.
5. Run the infusion slowly for the first 15 minutes, as with other blood products, and watch closely for any transfusion reactions. After this, the infusion rate can be moved up to 4 to 8 ml/kg per hour according to the patient’s tolerance (Rosenthal, 2004).
6. When the platelet pack is empty, close its roller clamp, open the normal saline solution, and flush the line to infuse all the platelets.
7. Document vital signs at 15 minutes into the infusion and again at the conclusion of the infusion.
FRESH FROZEN PLASMA
Fresh frozen plasma (also known as FFP, FP, or frozen plasma) contains all the components of plasma, including clotting factors and fibrinogen, of one unit of whole blood (American Red Cross, 2003b; Rosenthal, 2004).
Indications
1. To correct coagulation deficiencies for which specific factor concentrates are unavailable (American Red Cross, 2003b).
2. To correct a bleeding tendency of unknown cause or one associated with liver failure (American Red Cross, 2003b).
3. To correct coagulopathy and active bleeding, such as may occur during massive transfusion or in disseminated intravascular coagulation (American Red Cross, 2003b).
4. To reverse warfarin effect (American Red Cross, 2003b; Simmons, 2003).
Contraindications and Cautions
1. FFP must be transfused as soon as possible after it is thawed, and it must be infused in less than 4 hours (AABB, 2002). After thawing, FFP must be stored at 4° C and used within 24 hours (Duguid et al., 2004;).
2. A filter (170- to 200-micron) must be used for FFP transfusion (Duguid et al., 2004).
3. FFP is not indicated solely for volume expansion (American Red Cross, 2003b; Rosenthal, 2004).
4. FFP must be ABO compatible (Rosenthal, 2004).
5. Thawed FFP should be yellow or light green in color and clear in appearance (Rosenthal, 2004).
Procedural Steps
1. Identify the patient and the blood type and double check the unit of plasma with another person to ensure it is correct; it should be the same type. Document the double-checked data according to the institutional policy.
2. Inspect the bag for leaks and the color of the infusion (Duguid et al., 2004).
3. Prime the infusion set with normal saline.
4. Close the roller clamp on the normal saline solution and spike the plasma with the other tail of the Y set.
5. Open up the plasma, and regulate the drip rate.
6. Run the infusion slowly for the first 15 minutes, as with other blood products, and watch closely for any transfusion reactions.
7. Increase rate as prescribed.
8. Dosage is usually 10 to 15 ml/kg but may vary depending on clinical presentation (Duguid, et al., 2004).
9. Assess and document the vital signs after the initial 15 minutes of the infusion and again at the completion of the infusion.
CRYOPRECIPITATED ANTIHEMOPHILIAC FACTOR (CRYOPRECIPITATE)
Cryoprecipitate consists of clotting factor VIII (80 to 100 units), von Willebrand factor, fibrinogen (150 to 200 mg), and factor XIII suspended in 10 to 20 ml of plasma (American Red Cross, 2003a; Simpson, 2006).
Indications
1. To control bleeding by replacing clotting factors in the presence of the following (American Red Cross, 2003a; Simpson, 2006):
2. Factor VIII or factor XIII deficiency.
4. Hypofibrinogenemia, disseminated intravascular coagulation.
5. Cryoprecipitate is also used as fibrin glue surgical adhesives. This use is not FDA approved (American Red Cross, 2003a).
Contraindications and Cautions (AABB, 2002)
1. Cryoprecipitate should not be used as a substitute for FFP.
2. Compatibility testing not required, but ABO-compatible plasma should used when possible. Rh type does not need to be considered.
3. Volume of infusion is dependent on clinical situation.
4. Infuse only at room temperature.
5. Use only a plastic syringe, because factor VIII may bind to the surface of a glass syringe.
6. Cryoprecipitate must be given through a filter.
7. Transfusion reactions can occur.
8. Infuse immediately after thawing, with complete infusion within 4 hours.
Procedural Steps
1. Initiate an IV line with normal saline solution and Y-type blood set at a keep-vein-open (KVO) rate.
2. Inspect the bag for leaks and examine the solution.
3. Mix by inverting bag gently several times.
4. Hang the cryoprecipitate on the other tail of the Y-type blood set. Cryoprecipitate dosing is based on the patient’s plasma volume and the desired increase in factor VIII; multiple units are usually indicated (AABB, 2002).
5. Infuse slowly for the first 15 minutes and watch for a transfusion reaction. Then, adjust the drip rate to prescribed rate.
6. Flush the IV line with a normal saline solution after the cryoprecipitate is finished.
7. Assess and document the vital signs at 15 minutes into the infusion and immediately following the infusion.
Complications
1. Hemolytic-transfusion reactions occur due to incompatibility between donor and host (Simmons, 2003; Simpson, 2006) can be either immediate or delayed. Acute hemolysis can occur when recipient plasma antibodies react with the donor RBC antigens. Both acute and delayed hemolytic reactions are potentially life-threatening events.
2. Allergic reactions and fever occur in about 1 of every 100 units of blood. These usually occur as a result of the interaction of host antibodies with donor plasma proteins. An allergic reaction is most likely to occur with administration of whole blood or plasma because the plasma contains many antibodies and antigens.
3. Transfusion-related acute lung injury (TRALI) is the most common cause of transfusion-related deaths in recent years. The reaction activates the complement cascade and histamine release leading to pulmonary capillary permeability. This reaction occurs within 4 hours of the initiation of transfusions. Symptoms are the same as those for acute respiratory distress syndrome and may require intubation and mechanical ventilation (Wooldridge-King, 2005).
4. Graft-versus-host disease (GVHD) occurs when the blood product attacks the body’s tissues. This phenomenon is particularly worrisome in the immunocompromised patient (Simmons, 2003). Signs and symptoms include erythematous rash, liver function abnormalities, and profuse diarrhea. Treatment includes immunosuppression with corticosteroids, fluid and electrolyte replacement, and symptomatic management (Simpson, 2006).
5. Febrile nonhemolytic transfusion reactions. This reaction is reflected by any increase in temperature greater than 1° C (1.8° F) and may be accompanied by chills, headache, and flushing during or after transfusion. Treatment usually consists of stopping the transfusion of the IV line kept patent with normal saline. Administration of antipyretics and diphenhydramine may be ordered if allergic components are present. Blood samples should be drawn and crossmatching repeated (Simmons, 2003; Simpson, 2006).
6. Circulatory overload and pulmonary edema can occur, especially in older adults and patients with congestive heart failure. Close assessment of lung sounds, neck veins, and venous pressures is important in this patient population. Treatment may include administering diuretics and oxygen (Simmons, 2003; Simpson, 2006).
7. Hyperkalemia. As banked RBCs age, hemolysis occurs and potassium is released from the cells.
8. Hypocalcemia is another potential problem caused by a large amount of citrate-containing blood and blood products. Citrate chelates calcium and is a preservative used in many blood products. Normally, this problem is self-limiting and mild because citrate is quickly metabolized by the liver.
9. Infectious diseases, such as hepatitis, cytomegalovirus, Epstein-Barr virus, and human immunodeficiency virus may be transmitted via blood products. Bacterial contamination of donor blood is very rare, although immunosuppressive effects of blood transfusions may lead to increased infection rates after transfusion in immunocompromised patients, such as postoperative patients (Hall, Frawley, Griffith, Forestner, & Minei, 2003).
American Association of Blood Banks (AABB). Circular of information for the use of human blood and blood components, 2002. Retrieved September 30, 2006, from http://www.aabb.org/Documents/About_Blood/Circulars_of_Information/coi0702.pdf
American Red Cross, New England Region. Cryoprecipitate transfusion guidelines, 2003. Retrieved September 25, 2006, from http://www.newenglandblood.org/professional/plasmaguide.htm
American Red Cross, New England Region. Plasma transfusion guidelines, 2003. Retrieved September 25, 2006, from http://www.newenglandblood.org/professional/plasmaguide.htm
American Red Cross, New England Region. Platelet transfusion guidelines, 2003. Retrieved September 25, 2006, from http://www.newenglandblood.org/professional/plateletguide.htm
Baxter Healthcare Corporation. Albumin therapy: Ask an expert, 2003. Retrieved September 29, 2006, from http://www.albumintherapy.com/us/en/ask.html
Beyea S., Majewski C. Blood transfusion in the OR—Are you practicing safely? AORN Journal. 2003;78:1009–1010.
Duguid J., O’Shaughnessy D.F., Atterbury C., Maggs P.B., Murphy M., Thomas D., Yates S., Williamson L.M. Guidelines for the use of fresh frozen plasma, cryoprecpitate and cryosupernatant. The British Society for Haemotology. 2004;126:11–18.
Hall G.E., Frawley W.H., Griffith K.E., Forestner J.E., Minei J.P. Allogenic blood transfusion increases the risk of postoperative bacterial infection: A meta-analysis. Journal of Trauma. 2003;54:908–914.
Rosenthal K. Avoiding bad blood: Key steps to safe transfusions. Nursing made incredibly easy. 2004;25:20–28.
Simmons P. A primer for nurses who administer blood products. MEDSURG Nursing. 2003;12:184–191.
Simpson T.R. Transfusion therapy and blood and marrow stem cell transplantation. In: Nettina S.M., Mills E.J. Lippincott manual of nursing practice. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:962–978.
Wooldridge-King M. Transfusion reaction management. In: Lynn-McHale Wiegand D.J., Carlson K.K. AACN procedure manual for critical care. 5th ed. Philadelphia: Saunders; 2005:1024–1030.
PROCEDURE 73 Blood Filters
SeeProcedure 72for information about specific blood components.
INDICATION
To remove and screen aggregates found in stored blood (clots and debris from blood components) (Moore, 2005; Simpson, 2006). All blood products should be administered through a standard filter (170 to 260 microns), which removes gross fibrin clots from the blood product (AABB, 2002; Simpson, 2006). Blood products may be filtered before release from the blood bank or may be released from the blood bank accompanied by the appropriate filter (Simpson, 2006).
TYPES OF FILTERS
1. Standard filters: 170- to 260-micron filter. Used during transfusion of all blood products (red blood cells, platelets, fresh frozen plasma, cryoprecipitate) to remove macroaggregates (clots and fibrin that accumulate during storage) (Savoia, 2003).
2. Leukocyte-depletion filters: Used for patients who require leukocyte-depleted products due to the need for multiple transfusions of platelets. These filters remove 80% to 95% of white blood cells from the platelets and may be used when the component was not leukocyte reduced by the blood supplier (AABB, 2002; Simpson, 2006).
3. Microaggregate filters: An approximately 40-micron filter, which may be used to remove microaggregates (fibrin, platelets, and white blood cells) during red blood cell transfusions; these filters also aid in the removal of cytomegalovirus (Simpson, 2006).
CONTRAINDICATIONS AND CAUTIONS
1. A standard blood filter should be changed at least every 12 hours; change the filter earlier if the flow rate is diminished (Northover, 2006).
2. Do not use a filter for platelet infusion if it has previously filtered red blood cells. The trapped cells can block the passage of the platelets (Northover, 2006; Rosenthal, 2004).
3. Standard blood filters can be flushed with normal saline (Northover, 2006).
4. Each standard blood filter may be used for up to 4 units of blood (Northover, 2006).
5. Do not prime leukocyte-reduction filters with 0.9% normal saline as this will interfere with its filtering function; the infusion set itself should be primed with normal saline (Southern Health, 2003).
6. Leukocyte reduction filters can be used for 1 to 2 units of blood, according to manufacturer’s recommendations (Southern Health, 2003).
PROCEDURAL STEPS (Wooldridge-King, 2005)
1. Spike one tail of Y-type tubing with 0.9% normal saline and prime the tubing.
2. Fill the drip chamber, the filter area, and the tubing from the normal saline solution bag. Clamp the patient line and the solution line.
3. Ensure that the filter is completely saturated with fluid so that it can function properly; this step helps avoid damage to the constituents of the blood or blood components.
4. Close the roller clamp to the patient.
5. Spike the other tail of the Y-type tubing with the blood or blood component bag.
6. Close the patient clamp and hold the blood bag and the filter upright, with the blood bag slightly above the level of the normal saline solution. Open the blood clamp slowly to allow the blood to enter the drip chamber.
COMPLICATIONS
1. All blood and blood components must be delivered through a filter to prevent inadvertant delivery of clots and debris (AABB, 2002; Simpson, 2006).
2. During rapid resuscitation, the filter may clog, making it necessary to change the filter or tubing sooner than recommended to achieve the desired flow rates (Northover, 2006).
American Association of Blood Banks (AABB). Circular of information for the use of human blood and blood components, 2002. Retrieved February 17, 2007, from http://www.aabb.org/Documents/About_Blood/Circulars_of_Information/coi0702.pdf
Northover S. Administration of fresh blood products (clinical guideline). Melbourne, Australia: The Royal Children’s Hospital, 2006.
Moore J.M. Intravenous therapy. In: Newberry L., Criddle L.M. Sheehy’s manual of emergency care. 6th ed. St Louis: Mosby; 2005:99–122.
Rosenthal K. Avoiding bad blood: Key steps to safe transfusions. Nursing made incredibly easy. 2004;25:20–28.
Savoia H. Rationalising blood filters across Royal Children’s Hospital (memorandum). Victoria, Australia: The Royal Women’s Hospital, 2003.
Simpson T.R. Transfusion therapy and blood and marrow stem cell transplantation. In: Nettina S.N., ed. Lippincott manual of nursing practice. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:962–978.
Southern Health. Use of blood filters (clinical protocol). Clayton, Australia: Author, 2003.
Wooldridge-King M. Blood and blood component administration. In: Lynn-McHale Wiegand D.J., Carlson K.K. AACN procedure manual for critical care. 5th ed. Philadelphia: Saunders; 2005:991–999.
PROCEDURE 74 Massive Transfusion
There is no standard definition of massive blood transfusion. The definition used often in the literature is the replacement of 10 units of blood over a 24-hour period or the replacement of a patient’s circulating blood volume (Hardcastle, 2006). Other definitions may include the need for 4 units of packed red blood cells (PRBCs) within 4 hours with continued major bleeding, the transfusion of 50 units in 48 hours, or the tranfusion of 20 units in 24 hours and blood loss greater than 150 ml/min (Repine, Perkins, Kauvar, & Blackborne, 2006).
INDICATION
To treat severe hypovolemic shock secondary to blood loss. The goal of treatment is to restore circulating blood volume to a level that supports homeostasis, oxygen-carrying capacity, and oncotic pressure (Codner & Cinat, 2005).
CONTRAINDICATIONS AND CAUTIONS
1. There are no evidence-based guidelines on the procedural steps of massive transfusions (Hardcastle, 2006).
2. The patient’s religious beliefs may prohibit administration of blood or blood products.
3. Warm all fluids to be administered during a massive transfusion (Mikhail, 2004).
4. Using systolic blood pressure as a lone indicator to guide resuscitation may lead to underresuscitation. Vasonconstriction secondary to catecholamine release during times of shock, pain, and hypothermia may result in normal blood pressure values despite persistent hypovolemia (Schulman, 2005).
5. If more than 4 units of uncrossmatched type O blood are transfused, the patient should continue to receive type O blood due to subsequent difficulty with compatibility testing. Whenever possible, type-and-crossmatch specimens should be drawn upon presentation prior to administration of blood products (Mikhail, 2004).
6. Peripheral IV access should be obtained using a 16-G or larger-bore IV catheter. If peripheral access is not successful, obtain central venous access with a large bore (6 Fr to 9 Fr) introducer (Sweeney, 2003).
7. Interossesous (IO) access is a viable option in patients of all ages. It may not be possible to aspirate marrow from the IO site; however, fluids should run freely through the line without use of a pump (Sweeney, 2003).
8. Due to the risk of complications, subclavian and internal jugular veins should not routinely be used for massive transfusion (Sweeney, 2003).