- Structure and function of the thyroid hormones: T4 and T3
- Control of thyroid hormone production – role of the pituitary hormone, TSH
- Causes and consequences of overactive thyroid gland (hyperthyroidism)
- Causes and consequences of underactive thyroid gland (hypothyroidism)
- How measurement of blood concentration of T4, T3 and TSH aids in diagnosis of thyroid disorders
- Thyroid testing and pregnancy
The focus of this chapter is endocrinology, that branch of medical science which is concerned with organs or parts of organs responsible for production and secretion of hormones. These hormones or ‘biochemical messengers’ are transported in blood to distant target organs where they exert their various and specific effects. The thyroid gland is an endocrine organ responsible for production and secretion of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3). Of all endocrine disorders, those involving the thyroid are the most common. Thyroid disease affects around 3–5% of the adult population1 and there is evidence that incidence is increasing2, so that thyroid hormones are by far and away the most frequently measured hormones in clinical laboratories. In this chapter we consider how the laboratory contributes to diagnosis and monitoring of thyroid disorders.
Normal anatomy and physiology
Thyroid gland
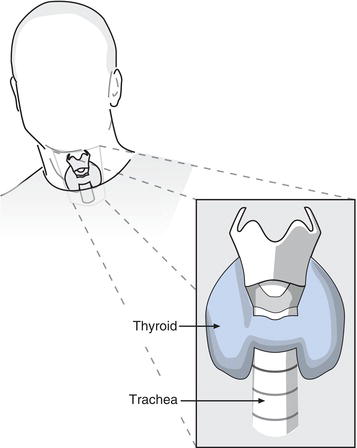
The thyroid gland (Figure 10.1), weighing around 20 g, is butterfly shaped and situated in the neck. The two lobes of the thyroid sit on either side of the trachea, just below the larynx and are connected by a bridge of tissue, the thyroid isthmus. Thyroid enlargement (goitre), a feature of many thyroid disorders may be visible as a swelling of the neck, or at least palpable on physical examination.
The gland is composed of two types of hormone producing cell, the bulk of the cells are so called follicle cells that produce the two thyroid hormones, T4 and T3. Interspersed between these cells are the parafollicular cells or C-cells that synthesis calcitonin, a hormone not considered here that is involved in calcium metabolism.
Function of thyroid hormones, T3 and T4
Thyroid hormones are delivered via the bloodstream to every part of the body and with few exceptions have an effect on the cells of all tissue types. Although T3 is the more potent of the two hormones, both increase the speed of many cellular metabolic reactions. For example, mobilisation and breakdown of body fat is increased in the presence of thyroid hormone, as is the speed of many reactions involved in the metabolism of carbohydrates and proteins. This overall stimulatory effect on the body’s metabolism determines that thyroid hormones are essential for normal growth and development, including sexual maturation. Specific effects of thyroid hormones are evident in relation to the heart and central nervous system. Cardiac output is influenced by the concentration of thyroid hormones in blood. Mental development from birth is dependent on adequate amounts of thyroid hormone: a deficiency at this time can lead not only to impaired growth but also to severe and irreversible mental retardation.
Thyroid hormone production
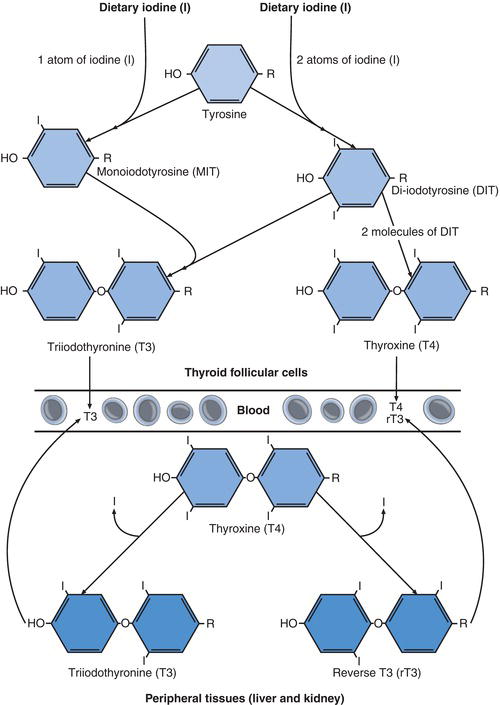
Dietary derived iodine is essential to thyroid hormone production and around 95% of the body’s iodine is concentrated in the thyroid. This element is present in sufficient amounts in a normal diet and absorbed to blood from the small intestine in the form of iodide. By an energy consuming process, iodide is removed from blood and ‘trapped’ in thyroid follicular cells, where it is required for production of T4 and T3. In the thyroid follicular cells iodide is oxidised to iodine and hormone synthesis begins with addition of iodine to the amino acid tyrosine, a process called iodination (Figure 10.2). Both the oxidising process and iodination are facilitated by the action of a key enzyme, thyroidal peroxidase (TPO).
Iodination results in monoiodotyrosine and diiodotyrosine. Two molecules of diiodotyrosine combine to form T4, which contains four iodine atoms; and one molecule of monoiodotyrosine combines with one molecule of diiodotyrosine to form T3, which contains three iodine atoms. Both T4 and T3 are released into the bloodstream from follicular cells, although 80% of circulating T3 is formed not in the thyroid but by enzymatic deiodination (removal of one molecule of iodine) of T4 in peripheral tissue, notably the liver and kidney. Two forms of T3 are formed in this way: physiologically active T3 and physiologically inactive reverse T3 (rT3); rT3 is a stereoisomer (structural mirror) of T3. More than 99% of both T4 and T3 that circulates in blood is bound to specific proteins, predominantly thyroxine binding globulin (TBG). In this protein bound form the hormones are inactive but serve as a reservoir or store of thyroid hormones. Less than 0.05% of total T3 and T4 in blood is present in a free (i.e. unbound to protein) and therefore physiologically active form. The nomenclature FT3 and FT4 is used to specifically identify these free thyroid hormone fractions.
Control of thyroid hormone production
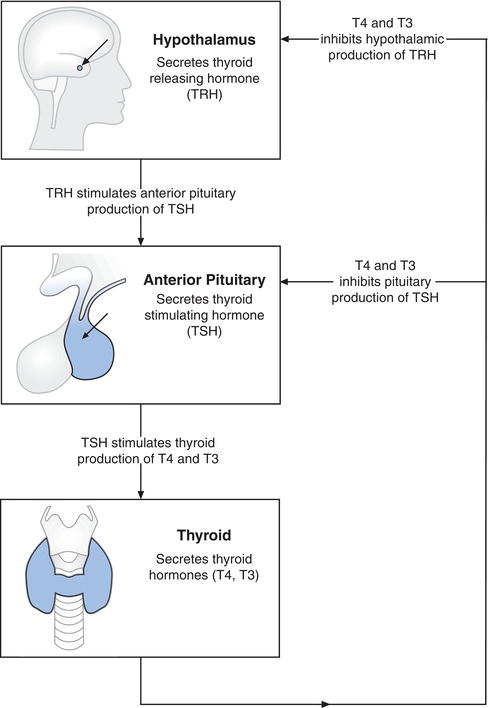
The maintenance of thyroid hormone blood concentration within normal limits is essential for good health so that production and secretion from the thyroid gland is finely controlled. This control depends on the pituitary gland, a pea sized organ located at the base of the brain (Figure 10.3). Among several hormones produced by this tiny gland is thyroid-stimulating hormone (TSH), also called thyrotropin. As its name implies, TSH stimulates production and secretion of thyroid hormones from the thyroid gland. The secretion of TSH is in turn controlled by thyrotrophin releasing hormone (TRH) secreted by the hypothalamus in the brain. Release of both TSH and TRH are controlled by the plasma concentration of circulating free thyroid hormone (FT4 and FT3). As thyroid hormone concentration falls, TRH and TSH secretion increases, stimulating the thyroid to secrete more thyroid hormone. Conversely as thyroid hormone concentration rises, TRH and TSH secretion decreases and consequently thyroid hormone production and secretion decrease. This continuous process of negative feedback maintains the amount of thyroid hormone in blood within normal limits.
Normal concentration of thyroid hormones in blood is dependent on:
- An adequate amount of dietary iodine for manufacture of thyroid hormones.
- A normally functioning thyroid gland.
- Adequate production of TSH and therefore normally functioning pituitary gland.
- Adequate production of TRH and therefore normally functioning hypothalamus.
Laboratory assessment of thyroid function
Patient preparation
No particular patient preparation is necessary.
Sample requirements
Around 5 ml of venous blood is required. The tests may be performed on blood plasma or blood serum; if local policy is to use plasma, then blood must be collected into a tube containing an anticoagulant (usually heparin) but if local policy is to use serum, blood must be collected into a plain tube (i.e. without any additive).
Request card information
Since drugs and pre-existing non-thyroid disease can affect interpretation it is important to record drug and brief clinical history. In addition to its role in the diagnosis of thyroid disorders, the test may also be used to monitor the effectiveness of therapy among those already diagnosed; it is important that the reason for requesting the test and dosage of any prescribed thyroxine replacement or antithyroid drugs are recorded.
In the laboratory
Two tests are used in the first line investigation of patients suspected of suffering thyroid disease:
- Thyroid stimulating hormone (TSH). The TSH test measures concentration in blood serum or plasma of the pituitary hormone TSH.
- Free thyroxine (FT4). The FT4 test measures the concentration in blood plasma or serum of free thyroxine (the biologically active fraction of total thyroxine that is not bound to protein).
A third test may be useful in particular circumstances:
- Free triiodothyronine (FT3). The FT3 test measures the concentration in blood serum or plasma of free triiodothyronine (the biologically active fraction of total triiodothyronine that is not bound to protein).
Before development of the highly sensitive laboratory methods required to detect the minute (picomole) concentration of free thyroid hormones (FT4 and FT3) present in blood, the only way of assessing thyroid function was to measure the concentration of total (free plus bound) T4 and T3. Although technically less demanding, measurement of total T4 and T3 (TT4, TT3) concentration is a less satisfactory means of assessing thyroid function, not least because results are affected by the plasma concentration of thyroid binding globulin (TBG), which varies in both healthy and disease states. The concentration of TBG has no effect on free hormone concentration, allowing clearer interpretation of results. Most laboratories now only offer the FT4 and FT3 tests but some may still be using the older TT4 and TT3 tests. For completeness these older tests will be included in the list of reference ranges below, but for the rest of the chapter attention will be focused on the now preferred tests, FT4 and FT3.
Interpretation of test results
Approximate reference ranges
| TSH | 0.3–4.5 mU/L |
| FT | 49–26 pmol/L |
| FT | 33.0–9.0 pmol/L |
| TT | 460–150 nmol/L |
| TT | 31.1–2.6 nmol/L |
Terms used in interpretation
| Euthyroid (ism) | normal thyroid activity. |
| Hyperthyroid (ism) | overactive thyroid gland. |
| Hypothyroid (ism) | underactive thyroid gland. |
| Goitre | enlargement of the thyroid gland. Depending on the cause, goitre may be a feature of euthyroidism, hyperthyroidism or hypothyroidism. |
| Thyrotoxicosis | the clinical syndrome which results from hyperthyroidism, often used as a synonym for hyperthyroidism. |
| Myxoedema | a clinical syndrome that includes accumulation of fluid (oedema) in skin tissue, which is associated with severe hypothyroidism. |
Causes of abnormal thyroid function test results
Over activity of the thyroid gland and associated increased production of thyroid hormones is called hyperthyroidism. In most instances this is due to disease of the thyroid gland itself, in which case the condition is known as primary hyperthyroidism. Very rarely hyperthyroidism occurs because a normally functioning thyroid gland is being over stimulated by inappropriately increased secretion of TSH from a diseased pituitary gland; this is known as secondary or central hyperthyroidism.
Similarly, under activity of the thyroid gland and concomitant reduced production of thyroid hormones, called hypothyroidism is most commonly due to disease of the thyroid gland (primary hypothyroidism) but can rarely result from decreased production of TSH by the pituitary gland (secondary or central hypothyroidism).
Primary hyperthyroidism
Graves’ disease is the most common cause of primary hyperthyroidism accounting for around 80% of cases. This is an autoimmune disease that is ten times more common among women than men; 0.5–2% of adult women have Graves’ disease1. Patients often report a family history of the condition; it is possible to inherit a predisposition. The thyroid gland of those suffering Graves’ disease is diffusely enlarged (smooth or diffuse goitre) and non-tender. The cause of the increased hormone production is abnormal thyroid stimulating antibodies, produced by the patient’s immune system, that act in the same way as TSH and stimulate the thyroid to produce thyroid hormones. Unlike TSH however, the production and action of these autoantibodies is not under negative feedback control, and continues despite rising thyroid hormone levels.
Three other less common conditions, toxic adenoma, toxic multinodular goitre and subacute thyroiditis, account for most of the remaining 20% of primary hyperthyroidism cases. Toxic adenoma is characterised by a single abnormal ‘nodule’ in the thyroid gland that secretes excessive thyroid hormone autonomously, whereas in toxic multinodular goitre the problem is many hypersecreting nodules. Leakage of thyroid hormone from follicular cells damaged by inflammation is the cause of the hyperthyroidism in those with subacute thyroiditis, a self-limiting, usually painful and debilitating condition that follows viral infection. The hyperthyroid phase is typically followed by a hypothyroid phase before recovery, which may take many months.
Amiodarone, a drug used to treat cardiac arrhythmias, is the most significant of a number of drugs that can cause hyperthyroidism. Whatever the cause, primary hyperthyroidism is characterised by increased concentration of thyroid hormone in blood and it is this feature which accounts for the many signs and symptoms (Table 10.1) that reflect the significance of thyroid hormones for all organ systems.
Since increased thyroid hormone production suppresses pituitary secretion of TSH (Figure 10.3), a reduction in serum or plasma TSH is an important diagnostic feature of primary hyperthyroidism. Measurement of TSH is the single most important biochemical test for investigation of those suspected of suffering primary hyperthyroidism because a diagnosis can almost always be excluded if TSH is within the reference range.
Table 10.1 Major signs and symptoms of hyperthyroidism.
| Thyroid hormone excess causes a general speeding of body metabolism and physiological process, resulting in any of the following signs and symptoms: |
|
* This is a feature of Graves’ disease only.
The blood results that would be expected in primary hyperthyroidism, no matter what the cause, are:
- Plasma/serum TSH concentration always reduced (undetectable in severe cases).
- Plasma/serum FT4 and FT3 concentrations increased.
- Occasionally FT4 is normal and only FT3 is increased (this is called T3 thyrotoxicosis).
Secondary (central) hyperthyroidism
Very rarely excessive thyroid hormone production is the result not of a problem within the thyroid but rather as a result of uncontrolled secretion of TSH due to disease (over activity) of the pituitary gland. For example, pituitary tumours secrete abnormally high amounts of TSH. In these rare cases, the thyroid is responding normally to abnormal increased stimulation. Typical blood results in secondary hyperthyroidism are:
- Plasma/serum TSH concentration raised.
- Serum FT4 and FT3 concentration raised.
Subclinical (mild) primary hyperthyroidism
Sometimes patient testing reveals reduced plasma concentration of TSH, indicating primary hyperthyroidism, in association with a normal (usually high normal) plasma concentration of FT4 and FT3, indicating euthyroidism. This pattern indicates that TSH production is partially suppressed by overactive thyroid, but there remains sufficient TSH to maintain thyroid hormone concentration within the reference range. It is not usually associated with symptoms because thyroid hormone concentration is not increased, so it is called subclinical hyperthyroidism. However it is not normal and represents a stage between normality and overt hyperthyroidism. Patients with sub-clinical hyperthyroidism are at greater than normal risk of developing overt hyperthyroidism, usually Graves’ disease in the long term, and should be offered thyroid testing every 6–12 months. In addition there is evidence that sub-clinical hyperthyroidism increases the risk of atrial fibrillation (an abnormal heart rhythm) in the elderly and reduced bone density (osteoporosis) in postmenopausal women3. Blood results to be expected in sub-clinical hyperthyroidism are:
- Plasma/serum TSH reduced.
- Plasma/serum FT4 and FT3 normal (often high normal).
Primary hypothyroidism
Like primary hyperthyroidism, primary hypothyroidism is ten times more common in women than men and, overall, primary hypothyroidism affects an estimated 1–2% of the UK population1. Incidence increases with age; close to 10% of elderly women are likely affected by hypothyroidism. Most cases of hypothyroidism are the result of slowly progressive autoimmune mediated destruction of thyroid tissue. There are two main forms: atrophic autoimmune thyroiditis and goitrous autoimmune thyroiditis (alternative name Hashimoto’s thyroiditis). In the first the thyroid is shrunken in size (atrophic) and function, and in the second the thyroid is enlarged (goitrous) – but still ‘shrunken’ in function. In both cases destructive autoantibodies are detectable in the patient’s serum and the same infiltration of the thyroid by inflammatory cells is evident; they are probably different stages of the same disease, with atrophy of the gland being the final stage.
The other major cause of primary hypothyroidism is two commonly employed treatment regimes for primary hyperthyroidism: thyroid surgery and irradiation of the thyroid gland. The aim of both treatments is destruction of thyroid tissue and an inevitable consequence of this destruction is increased long-term risk of primary hypothyroidism. Together, autoimmune destruction and treatment of hyperthyroidism account for more than 90% of all cases of primary hyperthyroidism.
Some drugs, notably lithium (Chapter 14) can cause hypothyroidism. Around 1 in 3500–4000 babies are born with a deficiency of thyroid hormone. All babies are screened at birth for this condition, which is called congenital hypothyroidism and considered further in Chapter 23. In some parts of the world a diet severely deficient of iodine and consequent reduced thyroid hormone production is a major cause of hypothyroidism.
Whatever the cause, primary hypothyroidism is associated with reduced thyroid hormone production and it is this hormone deficiency that accounts for symptoms in adults (Table 10.2). The normal response of the pituitary to reduced thyroid hormone production is increased production of TSH. An increased concentration of TSH in blood is the most important diagnostic feature of primary hypothyroidism.
Table 10.2 Major signs and symptoms of hypothyroidism.
| Thyroid hormone deficiency causes a general slowing of body metabolism and physiological process, resulting in any of the following signs and symptoms: |
|
The blood results that would be expected in primary hypothyroidism whatever the cause are:
- Plasma/serum TSH concentration always increased (usually >10 mU/L).
- Plasma/serum FT4 concentration reduced.
Secondary (central) hypothyroidism
Very rarely hypothyroidism is the result not of thyroid disease but of an inability to adequately stimulate a normal thyroid gland because of a deficiency of TSH. This occurs if there is trauma to or tissue damaging disease of the pituitary gland. Damage to the hypothalamus has the same effect. The blood results that would be expected in secondary hypothyroidism whatever the cause are:
- Plasma/serum TSH concentration reduced.
- Plasma/serum FT4 concentration reduced.
Sub-clinical (mild) hypothyroidism
Relatively frequently patient testing reveals raised serum TSH (indicating primary hypothyroidism) in association with serum FT4 within the reference range (indicating euthyroidism). This pattern indicates that although the thyroid gland is producing enough thyroid hormone to maintain concentration within the reference range, it is not producing sufficient to fully suppress TSH production. Thyroid hormone blood levels are only maintained within the reference range by virtue of the abnormally increased amount of TSH being released from the pituitary. Since thyroid hormone concentration is normal there are usually no symptoms, so the condition is called sub-clinical hypothyroidism. This represents a stage between normality and overt hypothyroidism. Patients with sub-clinical, sometimes called mild hypothyroidism are at long-term risk of developing overt hypothyroidism, especially if TSH is found to be particularly high (>10 mu/L), and should be offered annual thyroid testing.
The effect of non-thyroid illness on TSH, FT4 and FT3
The laboratory diagnosis of hyper and hypothyroidism whether sub-clinical or overt is rarely a problem in otherwise well patients, but interpretation of thyroid function test results is often more difficult in patients who are suffering acute illness. The term euthyroid sick syndrome or non-thyroidal illness syndrome is applied to those patients whose thyroid function tests (TSH, FT4 and FT3) are transiently abnormal due to acute illness. By definition the thyroids of these patients are working normally, the cause of the abnormal thyroid blood tests results is the acute illness. Euthyroid sick syndrome may reflect a protective adaptive response to acute illness or perhaps a maladaptive result of acute illness that impairs recovery from the illness. Either way, the practical clinical problem is that there is often difficulty in knowing if abnormal blood tests results are due to euthyroid sick syndrome or thyroid disease.
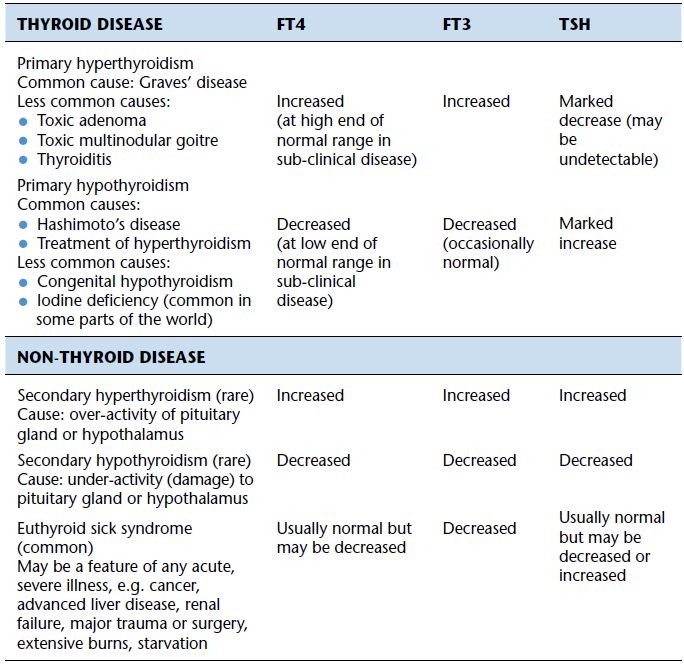
Table 10.3 Summary of typical changes to thyroid function test results in thyroid and non-thyroid disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree