Principles of Parenteral Administration
Sharon M. Weinstein
KEY TERMS
Acidifying Infusions
Alkalizing Fluid
Dehydration
Extracellular
Hemolysis
Hexoses
Hydrating Fluids
Hyperinsulinism
Hypertonic
Hypodermoclysis
Hypotonic
Interstitial
Intracellular
Isotonic
Osmolality
Turgor
Water Intoxication
PARENTERAL FLUIDS
To help enhance patient outcomes, knowledge of parenteral fluids is essential when delivering infusion therapy. This is particularly important because rapid and critical changes in fluid and electrolyte balance may be caused by infusates.
Until the 1930s, intravenous (IV) fluids consisted of dextrose and saline solutions. Little was known about electrolyte therapy. Today, however, more than 200 types of commercially prepared fluids are available. The great increase in their use leads to a more common occurrence of fluid and electrolyte disturbances. Moreover, with the increased administration of fluids in alternative care settings, nurses must know the chemical composition and the physical effects of the infusions they administer.
 PATIENT SAFETY
PATIENT SAFETYNurses have a legal and professional responsibility to know the
Normal amount of any IV infusion they administer
Desired and untoward effects of any IV infusion
Type of fluid, the amount, and the rate of flow, determined only after the physician/licensed independent practitioner (LIP) has carefully assessed the patient’s clinical condition
Intravenous Infusion
Today, methods of infusion have changed dramatically, and small-volume parenteral fluids may be administered as a secondary infusate or in a volume-controlled reservoir for electronic drug delivery.
An infusion is usually regarded as an amount of fluid > 100 mL designated to be infused parenterally because the volume must be administered over a long period. However, when medications are administered as a piggyback (secondary to and delivered with the initial infusion) small-volume (50 to 100 mL) parenteral infusion, a shorter period (usually 30 to 60 minutes) may be required, whereas volumes of 150 to 200 mL may require > 1 hour. Today, methodologies have changed to be consistent with the clinical needs of patients, and alternate delivery systems are readily available.
IV fluids are mistakenly referred to as IV solutions. The term solution is defined in the United States Pharmacopeia (USP) as a liquid preparation that contains one or more soluble chemical substances usually dissolved in water. Solutions are distinguished from injections, for example, because they are not intended for administration by infusion or injection. Moreover, methods of preparation may vary widely. The USP refers to parenteral fluids as injections, and methods of preparation must follow standards for injection. IV injections must meet the tests, standards, and all specifications of the USP applicable to injections. This includes quantitative and qualitative assays of infusions, including tests for pyrogens and sterility (McEvoy, 2007).
Particulate Matter
Each fluid container must be carefully examined to detect cracks. The fluid must be examined for cloudiness or particles. The final responsibility falls on the pharmacist and the nurse who administers the fluid. Tests to detect particulate matter, and standards for an acceptable limit of particles, have been established by the USP. A large-volume injection for singledose infusion meets the requirements of the test if it contains not more than 50 particles per milliliter that are ≥ 10.0 µm and not more than 5 particles per milliliter that are ≥ 25.0 m in effective linear dimension.
pH Value
The pH indicates hydrogen ion concentration or free acid activity in solution. All IV fluids must meet the pH requirements set forth by the USP. Most of these requirements call for a fluid that is slightly acid, usually ranging in pH from 3.5 to 6.2. Dextrose requires a slightly acid pH to yield a stable fluid. Heat sterilization, used for all commercial fluids, contributes to the acidity. It is important to know the pH of the commonly used IV fluids because it
may affect the stability of an added drug and cause the drug to deteriorate. The acidity of dextrose fluids has been criticized for its corrosive effect on veins.
may affect the stability of an added drug and cause the drug to deteriorate. The acidity of dextrose fluids has been criticized for its corrosive effect on veins.
Water
The cell wall separates the intracellular compartment from the extracellular compartment. The capillary endothelium and the arterial and venous walls divide the extracellular compartment into the intravascular and interstitial compartments. Water flows freely through cell and vessel calls and is distributed throughout these compartments. (Grocott, Mythen, & Gan, 2005).
Osmosis
Osmosis is the process by which a solvent, usually water, moves through a semipermeable membrane from a solution of lower concentration to a solution of higher concentration. The osmotic pressure exerted by particles in solution is determined by the number of particles per volume of fluid versus the mass or size of the particles (Phillips, 2005).
Capillary pressure has a tendency to force fluid and dissolved substances through the capillary pore into the interstitial spaces.
Tonicity
A change in water content causes cells to swell or shrink. Tonicity refers to the tension or effect that the osmotic pressure of a solution, with impermeable solutes, exerts on cell size due to water movement across the cell membrane.
Solutions are classified according to the tonicity of the fluid in relation to normal blood plasma. The osmolality of blood plasma is 290 mOsm/L. Fluid that approximates 290 mOsm/L is considered isotonic. IV fluids with an osmolality significantly > 290 mOsm (+50 mOsm) are considered hypertonic, whereas those with an osmolality significantly < 290 mOsm (−50 mOsm) are hypotonic.
Parenteral fluids usually range from approximately one-half isotonic (0.45% sodium chloride) to 5 to 10 times isotonic (25% to 50% dextrose). The tonicity of the fluid infused into the circulation affects fluid and electrolyte metabolism and may result in disastrous clinical disturbances (see Table 9-1 for more information on the effects of isotonic, hypertonic, and hypotonic fluids).
Knowing the osmolality of the infusion and the physical effect it produces alerts the nurse to potential fluid and electrolyte imbalances. The choice of veins used for an infusion is affected by the tonicity of the fluid; hyperosmolar fluids, for example, must be infused
through veins that carry a large blood volume to dilute the fluid and prevent trauma to the vessel.
through veins that carry a large blood volume to dilute the fluid and prevent trauma to the vessel.
TABLE 9-1 RESULTS OF INFUSION OF FLUIDS WITH DIFFERENT TONICITIES | ||||||||
|---|---|---|---|---|---|---|---|---|
|
The tonicity of the fluid also affects the rate at which it can be infused. Hypertonic dextrose infused rapidly may result in diuresis and dehydration.
Because of the direct and effective role osmolality plays in IV therapy, the nurse involved in administering IV fluids needs to be familiar with various terms and calculations. For instance, the osmotic pressure is proportional to the total number of particles in the fluid. The milliosmole (mOsm) is the unit that measures the particles or the osmotic pressure. By converting milliequivalents to milliosmoles, an approximate osmolality may be determined.
FLUIDS CONTAINING UNIVALENT ELECTROLYTES
Each milliequivalent is approximately equal to a milliosmole because univalent electrolytes, when ionized, carry one charge per particle. An injection of normal saline (0.9% sodium chloride) contains 154 mEq sodium and 154 mEq chloride per liter of fluid, making a total of 308 mEq/L, or approximately 308 mOsm/L.
FLUIDS CONTAINING DIVALENT ELECTROLYTES
Because each particle carries two charges when ionized, the milliequivalents per liter of fluid or the number of electrical charges per liter when divided by the charge per ion (2) gives the approximate number of particles or milliosmoles per liter. As an example, when 20 mEq magnesium sulfate is introduced into a liter of fluid, each ionized particle carries two charges. By dividing 20 mEq or 20 charges by 2, an approximate 10 particles or 10 mOsm/L is reached for each component, or 20 mOsm/L total.
The osmolality of electrolytes in solution may be accurately computed but involves using the atomic weight and the concentration of the given electrolytes in milligrams per liter. The methods for accurately computing the osmolality of an electrolyte, such as potassium (K), in solution follow:
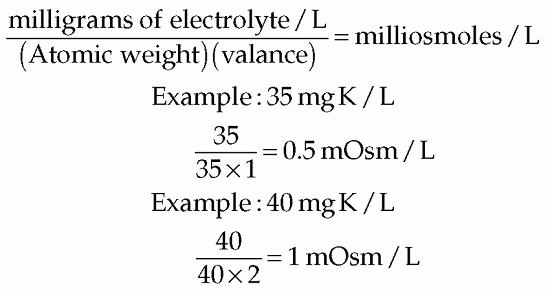
KINDS AND COMPOSITION OF FLUIDS
Various kinds of fluids are used for parenteral injections. These fluids are composed of dextrose in water, sodium chloride in water, sodium bicarbonate, ammonium chloride, and other substances.
Premixed Solutions
A significant number of solutions are available premixed. Premixed solutions have been sterilized following the admixture process, ensuring longer shelf life. The pH has already been adjusted to enhance stability, and the correct amount of medication has been added to the proper volume and type of solution.
Care should be taken in selecting premixed solutions when multiple types are available. For example, premixed solutions with potassium chloride are available in several concentrations. Other premixed solutions include heparin sodium, theophylline, lidocaine hydrochloride, and dopamine hydrochloride. Antibiotics are often premixed in small-volume parenterals. In addition to premixed solutions, some products allow the medication container to be connected to the IV solution. In general, premixed solutions tend to increase cost.
DEXTROSE IN WATER
When glucose is part of parenteral injections, it is usually referred to as dextrose, a designation by the USP for glucose of requisite purity. Dextrose is available in concentrations of 2.5%, 5%, 10%, 20%, and 50% in water. To determine the osmolality or the caloric value of a dextrose fluid, the nurse needs to know the total number of grams or milligrams per liter. Because 1 mL water weighs 1 g, and 1 mL is 1% of 100 mL, milliliters, grams, and percentages can be used interchangeably when calculating solution strength. Thus, dextrose 5% in water equals 5 g dextrose in 100 mL, or 50 g dextrose in 1 L. The metabolic effects of dextrose are listed in Box 9-1.
CALORIES
Hexoses (glucose or dextrose and fructose) do not yield 4 calories per gram, as do dietary carbohydrates (e.g., starches). Each gram of hydrous or anhydrous dextrose provides approximately 3.4 or 3.85 calories, respectively. One liter of 5% glucose infusion yields 170 calories; 1 L of 10% glucose infusion yields 340 calories (USP, 2012).
TONICITY OF DEXTROSE 5% IN WATER
Dextrose 5% in water is considered an isotonic fluid because its tonicity approximates that of normal blood plasma, or 290 mOsm/L. Because dextrose is a nonelectrolyte and the total number of particles in solution does not depend on ionization, the osmolality of dextrose
fluids is determined differently from that of electrolyte fluids. One millimole (one formula weight in milligrams) of dextrose represents 1 mOsm (unit of osmotic pressure). One millimole of monohydrated glucose is 198 mg, and 1 liter of dextrose 5% in water contains 50,000 mg. Thus:
fluids is determined differently from that of electrolyte fluids. One millimole (one formula weight in milligrams) of dextrose represents 1 mOsm (unit of osmotic pressure). One millimole of monohydrated glucose is 198 mg, and 1 liter of dextrose 5% in water contains 50,000 mg. Thus:
BOX 9-1 METABOLIC EFECTS OF DEXTROSE
Provides calories for essential energy
Improves hepatic function because glucose is converted into glycogen by the liver
Spares body protein (prevents unnecessary breakdown of protein tissue)
Prevents ketosis or excretion of organic acid (which may occur when fat is burned by the body without an adequate supply of glucose)
Stored intracellularly in the liver as glycogen, causes the shift of potassium from the extracellular to the intracellular fluid compartment (Note: This effect is desired as treatment for hyperkalemia. It is achieved by infusing dextrose and insulin.)
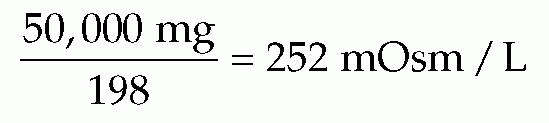
pH VALUE
The USP requirement for the pH of dextrose fluids is 3.5 to 6.5. This broad range may at times contribute to an incompatibility in one bottle of dextrose and not in another when an additive is involved.
INDICATIONS FOR USE
Dextrose fluids are used for patients with dehydration, hyponatremia, and hyperkalemia. They are also vehicles for drug delivery and nutrition.
DEHYDRATION
In cases of dehydration, dextrose 2.5% in water and dextrose 5% in water provide immediate hydration for medical as well as surgical patients. Dextrose 5% in water is considered isotonic only in the bottle. Once infused into the vascular system, the dextrose is rapidly metabolized, leaving water. The water decreases the osmotic pressure of the blood plasma and invades the cells, providing immediately available water to dehydrated tissues.
HYPERNATREMIA
If the patient is not in circulatory difficulty with extracellular expansion, dextrose 5% in water may be administered to decrease the concentration of sodium.
MEDICATION ADMINISTRATION
Many of the drugs for IV therapy are added to infusions of dextrose 5% in water.
NUTRITION
Concentrations of dextrose 20% and 50% in conjunction with electrolytes provide long-term nutrition. Insulin is frequently added to prevent overtaxing the islet tissue of the pancreas.
 PATIENT SAFETY
PATIENT SAFETYBecause the kidneys do not store potassium, prolonged fluid therapy with electrolytefree fluids may result in hypokalemia. This happens when cells are anabolized by the metabolism of glucose and potassium shifts from the extracellular to the intracellular fluid. Conversely, when renal function is impaired, IV potassium should be used cautiously. Remain alert for signs of hyperkalemia. If such signs develop, notify the physician/LIP and be prepared to replace fluid components with more appropriate electrolytes.
HYPERKALEMIA
Infusions of dextrose in high concentration with insulin cause anabolism (buildup of body cells), which results in a shift of potassium from the extracellular to the intracellular compartment, thereby lowering the serum potassium concentration.
DANGERS OF USE
Among abnormalities resulting from infusion of dextrose are dehydration, hypokalemia, hyperinsulinism, and water intoxication.
DEHYDRATION
Osmotic diuresis occurs when dextrose is infused at a rate faster than the patient’s ability to metabolize it. A heavy load of nonmetabolized glucose increases the osmolality of the blood and acts as a diuretic; the increased solute load requires more fluid for excretion. A normal, healthy person with a urine specific gravity of 1.029 to 1.032 requires 15 mL water to excrete 1 g solute, whereas people with poor kidney function or low concentrating ability of the kidneys require much more water to excrete the same amount of solute (Metheny, 2012).
HYPERINSULINISM
A rapid infusion of hypertonic carbohydrate solutes may result in hyperinsulinism. In response to a rise in blood glucose level, extra insulin pours from the beta cells of the pancreatic islets in an attempt to metabolize the infused carbohydrate. Terminating the infusion may leave excess insulin in the body, resulting in symptoms such as nervousness, sweating, and weakness caused by the severe hypoglycemia that may be induced. Typically, after infusion of hypertonic dextrose, a small amount of isotonic dextrose is administered to cover the excess insulin (Metheny, 2012).
WATER INTOXICATION
An imbalance resulting from an increase in the volume of the extracellular fluid from water alone is known as water intoxication. Prolonged infusions of isotonic or hypotonic dextrose in water may cause this condition, which is compounded by stress and leads to inappropriate release of antidiuretic hormone (ADH) and fluid retention. The average adult can metabolize water at a rate of approximately 35 to 40 mL/kg/day, and the kidney can safely metabolize only approximately 2,500 to 3,000 mL/day in an average patient receiving IV therapy (Metheny, 2012). Under stress, the patient’s ability to metabolize water decreases (Box 9-2).
BOX 9-2 POTENTIAL CONSEQUENCES OF INFUSING SPECIFIC FLUIDS
Cellular dehydration may result from excessive infusions of hypertonic fluids.
Water intoxication results when hypotonic fluid is infused beyond the patient’s tolerance for water.
Circulatory overload can result from isotonic fluid administration.
ADMINISTRATION
Isotonic dextrose may be administered through a peripheral vein. Hyperosmolar fluids, such as dextrose 50% in water, should be infused into the superior vena cava through central venous access. Hypertonic dextrose administered through a peripheral vein with small blood volume may traumatize the vein and cause thrombophlebitis. Infiltration can result in tissue necrosis.
Sodium-free dextrose injections should not be administered by hypodermoclysis (direct administration into subcutaneous tissue to enhance hydration). Dextrose fluids, by attracting body electrolytes in the pooled area of infusions, may cause peripheral circulatory collapse and anuria in sodium-depleted patients.
Electrolyte-free dextrose injections should not be used in conjunction with blood infusions. Dextrose mixed with blood causes hemolysis of the red blood cells.
The amount of water required for hydration depends on the clinical condition and the needs of the patient. The average adult patient requires 1,500 to 2,500 mL water each day. In patients with prolonged fever, however, the water requirement depends on the degree of temperature elevation. For example, the 24-hour fluid requirement for a patient with a body temperature between 101°F and 103°F increases by at least 500 mL; the fluid requirement for a prolonged temperature above 103°F increases by at least 1,000 mL.
The rate of administration depends on the patient’s condition and the purpose of therapy. When the infusion is used to supply calories, the rate must be slow enough to allow complete metabolism of the glucose (0.5 g/kg/hour in normal adults). The maximum rate usually should not exceed 0.8 g/kg/hour. When the infusion is used to produce diuresis, the rate must be fast enough to prevent complete metabolism of the dextrose, thereby increasing the osmolality of the extracellular fluid.
Isotonic Sodium Chloride Infusions
Sodium chloride injection (0.9%), USP (normal saline), contains 308 mOsm/L (sodium, 154 mEq/L; chloride, 154 mEq/L). It has a pH between 4.5 and 7.0 and is usually supplied in volumes of 1,000, 500, 250, and 100 mL. The term normal (or physiologic) is misleading because the chloride in normal saline is 154 mEq/L, compared with the normal plasma chloride value of 103 mEq/L, whereas the sodium is 154 mEq/L, or approximately 9% higher than the normal plasma value of 140 mEq/L. Because normal saline lacks the other electrolytes present in plasma, the isotonicity of the fluid depends on the sodium and chloride ions, resulting in a higher concentration of these ions (USP, 2012).
INDICATIONS FOR USE
Normal sodium chloride injection is indicated for the following:
Extracellular fluid replacement when chloride loss is relatively greater than or equal to sodium loss.
Treatment of metabolic alkalosis in the presence of fluid loss; the increase in chloride ions provided by the infusion causes a compensatory decrease in the number of bicarbonate ions.
Sodium depletion. When there is an extracellular fluid volume deficit accompanying the sodium deficit, an isotonic solution of sodium chloride is used to correct the deficit (Metheny, 2012).
Initiation and termination of blood transfusions. When 0.9% sodium chloride is used to precede a blood transfusion, the hemolysis of red blood cells, which occurs with dextrose in water, is avoided.
 PATIENT SAFETY
PATIENT SAFETYSodium chloride 0.9% solution (normal saline) provides more sodium and chloride than the patient needs. Marked electrolyte imbalances have resulted from the almost exclusive use of normal saline. Hypernatremia, acidosis, and circulatory overload may result when normal saline is administered in excess of the patient’s tolerance.
DANGERS OF USE
An adult’s dietary requirement for sodium is approximately 90 to 250 mEq/day, with a minimum requirement of 15 mEq and a maximum tolerance of 400 mEq (United States Pharmacopeia [USP], 2012). When 3 L of 0.9% sodium chloride or dextrose 5% in 0.9% sodium chloride is administered, the patient receives 462 mEq sodium (154 mEq/L), a level that exceeds normal tolerance.
HYPERNATREMIA
Infusion of saline during a period of sodium retention—for example, during stress—can result in hypernatremia. The danger of hypernatremia increases in the elderly, in patients with severe dehydration, and in patients with chronic glomerulonephritis; these patients require more water to excrete the salt than do patients with normal renal function. Isotonic saline does not provide water but requires most of its volume for the excretion of salt.
ACIDOSIS
One liter of 0.9% sodium chloride solution contains one third more chloride than is present in the extracellular fluid. When infused in large quantities, the excess chloride ions cause a loss of bicarbonate ions and result in acidosis.
HYPOKALEMIA
Infusion of saline increases potassium excretion and at the same time expands the volume of extracellular fluid, further decreasing the concentration of the extracellular potassium ion.
CIRCULATORY OVERLOAD
Continuous infusions of isotonic fluids expand the extracellular compartment and lead to circulatory overload.
REQUIREMENTS
In an average adult, the daily requirements of sodium chloride are met by infusing 1 L of 0.9% sodium chloride or 1 to 2 L of 4.5% sodium chloride, but the dosage depends on the patient’s age, weight, clinical condition, and fluid, electrolyte, and acid-base status.
Dextrose 5% in 0.9% Sodium Chloride
Dextrose 5% in 0.9% sodium chloride (normal or isotonic saline solution), which contains 252 mOsm dextrose (chloride, 154 mEq/L; sodium, 154 mEq/L), has a pH of 3.5 to 6.0 and is available in volumes of 1,000, 500, 250, and 150 mL. When normal saline is infused, the addition of 100 g dextrose prevents both the formation of ketone bodies and the increased demand for water the ketone bodies impose for renal excretion. The dextrose prevents catabolism and, consequently, loss of potassium and intracellular water.
INDICATIONS FOR USE
Isotonic saline with dextrose injection is indicated for the following:
Temporary treatment of circulatory insufficiency and shock caused by hypovolemia in the immediate absence of a plasma expander
Early treatment along with plasma or albumin for replacement of loss caused by burns
Early treatment of acute adrenocortical insufficiency
DANGERS OF USE
The hazards are the same as those for normal saline injection (see preceding section).
Dextrose 10% in 0.9% Sodium Chloride
Dextrose 10% in 0.9% sodium chloride contains 504 mOsm/L dextrose (sodium, 154 mEq/L; chloride, 154 mEq/L), has a pH of 3.5 to 6.0, and is usually supplied in volumes of 1,000 and 500 mL.
INDICATIONS FOR USE
This fluid is used as a nutrient and an electrolyte (sodium and chloride) replenisher.
ADMINISTRATION
Dextrose 10% in 0.9% sodium chloride, because of its hypertonicity, must be administered IV, preferably through a wide vein to dilute the fluid and reduce the risk of trauma to the vessel. Close observation and precautions are necessary to prevent infiltration and damage to the tissues.
Hypertonic Sodium Chloride Infusions
These infusions include 3% sodium chloride (sodium, 513 mEq/L; chloride, 513 mEq/L) and 5% sodium chloride (sodium, 850 mEq/L; chloride, 850 mEq/L).
INDICATIONS FOR USE
Infusion of hypertonic sodium is indicated for
Severe dilutional hyponatremia (water intoxication). Hypertonic sodium chloride, on infusion, increases the osmotic pressure of the extracellular fluid, drawing water from the cells for excretion by the kidneys.
Severe sodium depletion. Infusions of hypertonic saline replenish sodium stores. An estimate of the sodium deficit can be made by taking the difference between the normal sodium concentration and the patient’s current sodium concentration and multiplying the difference by 60% of the body weight in kilograms; sodium depletion is based on total body water and not on extracellular fluid.
ADMINISTRATION
Hypertonic sodium chloride injection must be administered with great caution to prevent pulmonary edema. Cautions include frequent reevaluation of the patient’s clinical and electrolyte status during administration. A 3% or 5% solution of sodium chloride is used to correct the deficit, providing the fluid volume is normal or excessive; the amount of sodium administered depends on the sodium deficit in the plasma (Metheny, 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
