Pharmacology Applied to Infusion Therapy
Lindsey M. Ladell
Sherry A. Tennies
KEY TERMS
Double Decomposition
Drug Concentration
Half-life
Hydrolysis
Incompatibility
Laminar Flow
Leaching
Oxidation
pH
Pharmacokinetics
Plasma Concentration
Precipitation
Receptor Site
Reduction
Therapeutic Index
ADMINISTERING DRUG THERAPY BY THE INTRAVENOUS ROUTE
The administration of medications via the intravenous (IV) route is common across health care settings. The nurse whose patients receive IV drug therapy needs to be well versed in the advantages and disadvantages of drug therapy delivered by the IV route. The nurse also needs to be a skilled practitioner who can administer IV drugs safely, monitor the patient’s response to therapy, instruct the patient regarding the prescribed medication therapy, and prevent potential adverse events. Nurses must have a comprehensive understanding of IV drug therapy to ensure patient safety and quality patient outcomes.
 PATIENT SAFETY
PATIENT SAFETYNurses are in a unique position to improve IV drug administration due to their proximity to the patient, insight to identify potential problems and to be a part of safety solutions.
This chapter is intended to expand the nurse’s knowledge about pharmacology as it relates to the safe preparation and delivery of IV drug therapy. The Institute of Medicine (IOM), in its hallmark report, demonstrated that the most common type of medical error is related to medication administration (IOM, 1999); thus, each section will address related patient safety matters. The first section offers a review of infusion therapy, including advantages and disadvantages of the IV route. The second section addresses patient considerations during IV medication delivery. The third section discusses pharmacokinetics and selected parameters for IV drugs. The fourth section details drug dosage and flow rate calculations.
ADVANTAGES OF THE INTRAVENOUS ROUTE
The IV route of drug administration offers pronounced advantages:
Offers an alternate route for medication administration in patients who are unable to take medications orally or for those who need medications that cannot be absorbed by any other route
Allows drugs to be administered to a patient who cannot tolerate fluids and drugs by the gastrointestinal route
Allows for the capacity to control the rate of administration of medications and the ability to slow or stop drug delivery at once if sensitivity reactions occur
Provides the route of administration for drugs that would not otherwise be able to be absorbed by the gastrointestinal route due to large molecular size or due to instability of the medication in the presence of gastric juices destroying the drug
Provides the route of administration for medications that have irritating properties and cause pain and trauma when given intramuscularly or subcutaneously
Provides rapid drug action via the vascular system since the drug bypasses processing in the gastrointestinal system and circulates directly in the bloodstream
Offers better control over the rate of administration of drugs; prolonged action can be provided by administering a dilute infusion intermittently or over a prolonged period
Understanding the basics of pharmacokinetics, pharmacodynamics, and pharmacotherapeutics provides an insight into how the IV route offers rapid drug action and better control over the duration of action.
 PATIENT SAFETY
PATIENT SAFETYEven with the advantages of the IV route, there remains the potential for infection from the insertion site, catheter, or infusate. On a daily basis, review the need for IV access (CDC, 2011).
Pharmacokinetics and Selected Parameters for Intravenous Drugs
Pharmacokinetics refers to the way in which a medication reacts in the body and includes how a drug is absorbed, distributed, metabolized, and eliminated by the body. The pharmacokinetic basis of therapeutics relies on various factors, such as the drug’s route and frequency of administration, to achieve and maintain a proper drug concentration at the receptor site, where the drug exerts its action. If an insufficient amount of drug reaches this site of action, the drug may appear to be ineffective, and the therapy may therefore be discontinued. Conversely, the typically appropriate dosage of a drug may produce toxicity, and therefore therapy may be discontinued simply because excessive amounts are in the body. An awareness of how drug pharmacokinetics affects the body is an essential part of preventing or lessening potential toxicity. Factors influencing the pharmacokinetics of drugs are presented in Box 18-1. Some aspects of pharmacokinetics that are particularly relevant to IV drug administration are therapeutic index, plasma concentration, and drug distribution.
THERAPEUTIC INDEX
The therapeutic index is the margin between a drug’s therapeutic and toxic concentrations at the receptor site. Some medications, such as warfarin, are considered to have a narrow therapeutic index, meaning that the margin between therapeutic and toxic concentrations is small. Pharmacokinetic models describe and predict drug amounts and concentrations in various body fluids and the changes in these quantities over time.
PLASMA CONCENTRATIONS
Plasma concentration is a measurement representing a drug that is bound to plasma protein plus a drug that is unbound or free. Two factors determine the degree of plasma protein binding. The first is the binding affinity of the drug for the plasma proteins; the second is
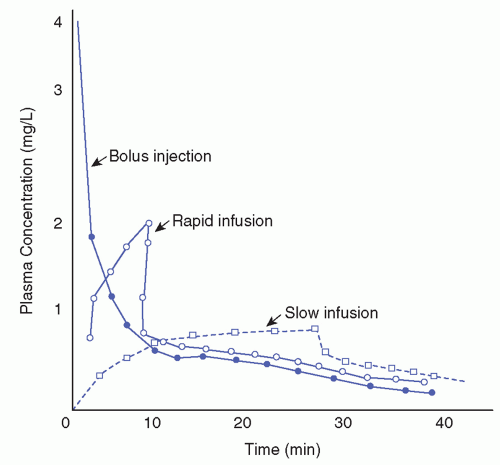
the number of binding sites available or the concentration of plasma protein. An acidic drug, such as salicylate or phenytoin, may be bound to the protein, albumin. A basic drug, such as lidocaine or quinidine, may be bound to serum globulins. Calculated plasma concentrations may assist the provider in evaluating the effectiveness or potential for toxicity of the therapy. Figure 18-1 shows concentration-time curves after IV drug injection at three different rates.
the number of binding sites available or the concentration of plasma protein. An acidic drug, such as salicylate or phenytoin, may be bound to the protein, albumin. A basic drug, such as lidocaine or quinidine, may be bound to serum globulins. Calculated plasma concentrations may assist the provider in evaluating the effectiveness or potential for toxicity of the therapy. Figure 18-1 shows concentration-time curves after IV drug injection at three different rates.
BOX 18-1 FACTORS INFLUENCING THE PHARMACOKINETICS OF DRUGS AND PATIENT RESPONSE
Patient Characteristics
Age, sex, body weight, dietary habits, smoking habits, alcohol consumption, coingestion of other drugs, other treatment protocols
Disease States
Hepatic disease, renal disease, congestive heart failure, infection, fever, shock, severe burns
THERAPEUTIC CONCENTRATION AND LOADING DOSE
The drug concentration level may be calculated after the dosage regimen is initiated to ensure that the selected dosage produces the drug concentration desired. It may also be measured periodically to ensure that the dosage continues to produce the desired target concentration or to determine if the therapeutic regimen is failing. Table 18-1 highlights therapeutic drug concentrations and toxic values.
The loading dose is the initial dose of drug ordered and is used to decrease the time it takes for a drug to reach a target or the therapeutic, concentration. Loading doses are not always practical or safe but necessary at times. For example, when administering vancomycin, the first dose may be given as a loading dose to assure that the drug concentration reaches the appropriate therapeutic level to kill bacteria.
DRUG HALF-LIFE
The term half-life denotes the time required for the plasma concentration of a drug or the total amount of drug in the body to decline by one half. The half-life of a drug depends on its volume of distribution (the distribution between the plasma and the various other fluids and tissues, and the actual nature of distribution and clearance). Upon discontinuation of
a medication, the drug concentration is reduced by 50% after one half-life; it is reduced by 75% after two half-lives; it is reduced by 87.5% after three half-lives; and the drug concentration is reduced by 93.75% after four half-lives.
a medication, the drug concentration is reduced by 50% after one half-life; it is reduced by 75% after two half-lives; it is reduced by 87.5% after three half-lives; and the drug concentration is reduced by 93.75% after four half-lives.
TABLE 18-1 SELECTED THERAPEUTIC DRUG CONCENTRATIONS AND TOXIC VALUES | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
DISADVANTAGES OF THE INTRAVENOUS ROUTE
Despite the advantages of the IV route, there are certain actual or potential hazards. They include the following:
Therapeutic incompatibilities: may occur when one or more drugs are added to the IV solution, including antagonism, synergism, and potentiation (Turner & Hankins, 2010).
Anaphylaxis: a hypersensitive reaction induced by preliminary drug injection.
Speed shock: an adverse systemic reaction to a substance rapidly injected into the bloodstream.
Vascular irritations and subsequent problems: including phlebitis, infiltration, extravasation, infection, and air embolism.
Rapid onset of action: inability to recall a drug once it enters the bloodstream.
Precipitate formation: may occur when one or more drugs are added to a parenteral solution. The precipitate does not always form when the solution is prepared, which increases the challenge of IV administration. Some drugs that are stable for a limited time degrade and may or may not precipitate as they become less therapeutically active. If administered IV, fluids containing insoluble matter carry the potential danger of embolism, myocardial damage, and harmful effects on other organs such as the liver and the kidneys.
Incompatibilities
The number of possible drug combinations is overwhelming, as is the potential for incompatibility. Incompatibility is the undesired reaction occurring between the drug and the solution, the container, or another drug (Turner & Hankins, 2010). Manufacturers provide an ever-increasing number of new drug products for the treatment of disease processes.
Compounding drugs for IV administration is usually performed as a component of a hospital-based or home infusion pharmacy. Today, admixture programs have expanded their service base to provide compounding services for extended care, ambulatory care, and any clinical setting in which infusion therapies are delivered.
Compounding drugs for IV administration is usually performed as a component of a hospital-based or home infusion pharmacy. Today, admixture programs have expanded their service base to provide compounding services for extended care, ambulatory care, and any clinical setting in which infusion therapies are delivered.
All people responsible for mixing and compounding IV drugs must be alert to the hazards involved. Compatibility charts are provided through admixture services to alert the nurse to potential problems, particularly when additions to infusions in progress are made on a nursing unit or added in a patient’s residence.
A factor related to compatibility is the composition of the solution container. Box 18-2 lists drugs affected by increased adsorption (adhesion to the surfaces of substances with which they are in contact) when infused in polyvinyl chloride (PVC) containers (Trissel, 2013). Factors affecting the compatibility of drugs include order of mixing, quantity of the drug and solution, room temperature, and light. Incompatibilities are not always obvious to the naked eye. They may be classified as physical, chemical, or therapeutic and are described in Box 18-3.
Leaching is the release of di(2-ethylhexyl)phthalate (DEHP) from PVC containers into the medication in solution. DEHP has a wide range of toxic effects, especially in male neonates where the infant’s reproductive system can be compromised. Products like lipid emulsions and cyclosporine exacerbate leaching.
CHEMICAL
The most common incompatibilities are the result of certain chemical reactions (FDA, 2012)—for example, hydrolysis is the process in which water absorption causes decomposition of a compound. In preparing solutions of salt, the nurse should understand that certain salts, when placed in water, hydrolyze, forming a strong acid and a weak base or a weak acid and a strong base. Because pH is a significant factor in the solubility of drugs, the increased acidity or alkalinity from hydrolysis of a salt may result in an incompatibility if another drug is added. For example, the acid salt sodium bicarbonate when placed in water
hydrolyzes to form a strong alkali (sodium hydroxide) and a weak and unstable acid (carbonic acid). Many organic acids are known as weak acids because they ionize only slightly.
hydrolyzes to form a strong alkali (sodium hydroxide) and a weak and unstable acid (carbonic acid). Many organic acids are known as weak acids because they ionize only slightly.
BOX 18-2 DRUGS WITH INCREASED ADSORPTION WHEN INFUSED USING PVC CONTAINERS
Diazepam
Hydralazine (Apresoline)
Insulin
Lorazepam
Nitroglycerin
Phenothiazine tranquilizers (Thorazine, Compazine)
Thiopental sodium
Urokinase
Vitamin A acetate (nonpalmitate form)
Warfarin sodium
BOX 18-3 TYPES OF INCOMPATIBILITIES
Physical—Those in which an undesirable change is physically observed, such as sodium bicarbonate and calcium chloride forming an insoluble precipitate (see http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf)
Chemical—Those occurring in the molecular structure or pharmacologic properties of a substance (note: They may or may not be physically observed), such as penicillin and ascorbic acid lowering the pH
Therapeutic—Those in which an undesirable reaction results from the overlapping effects of two drugs given together or closely together, such as penicillin and tetracycline inhibiting the bactericidal effect of penicillin
Reduction is the process whereby one or more atoms gain electrons at the expense of some other part of the system (ASHP, 2013).
Oxidation is the corresponding loss of electrons occurring when reduction takes place. Antioxidants are often used as preservatives to prevent oxidation of a compound.
Double decomposition is the chemical reaction in which ions of two compounds change places and two new compounds are thus formed (ASHP, 2013). A great many salts act by double decomposition to form other salts, and this process accounts for a large number of incompatibilities. For example, calcium chloride is incompatible with sodium bicarbonate; the double decomposition results in the formation of the insoluble salt, calcium carbonate.
PHYSICAL
Precipitation may occur in some parenteral solutions; some commonly prescribed drugs precipitate when added to IV solutions. Differences in the physical and chemical properties of each of these solutions may affect the stability of any drug introduced.
Moreover, a compound that is soluble in one solution may precipitate in another. Sodium ampicillin deteriorates in acid solutions. This drug, when added to isotonic sodium chloride at a concentration of 30 mg/mL, loses < 10% activity in 8 hours. However, when it is added to 5% dextrose in water, usually a more acid solution, its stability is reduced to a 4-hour period.
PH AND STABILITY
Because pH plays an important role in drug solubility, stability, and compatibility, a definition is in order. The term pH stands for the degree of concentration of hydrogen ions or the acidity of the solution. The weight of hydrogen ions in 1 L pure water is 0.0000001 g, which is numerically equal to 10− 7. For convenience, the negative logarithm is used. Because it is at this concentration that the hydrogen ions balance the hydroxyl ions, a pH of 7 is neutral. Each unit decrease in pH represents a 10-fold increase in hydrogen ions.
The greatest number of drug incompatibilities may be produced by changes in pH (ASHP, 2013). For example, precipitation occurs when a compound is insoluble in solution.
The degree of solubility often varies with the pH. A drastic change in the pH of a drug when added to an IV solution suggests an incompatibility or a decrease in stability.
The degree of solubility often varies with the pH. A drastic change in the pH of a drug when added to an IV solution suggests an incompatibility or a decrease in stability.
Solutions with a high pH appear to be incompatible with solutions with a low pH and may form insoluble free acids or free bases. A chart denoting the pH of certain drugs and certain solutions to be used as a vehicle is helpful in warning of potential incompatibilities. A broad pH range (3.5 to 6.5) of dextrose solutions is allowed by the United States Pharmacopeia (USP). A drug may be stable in one bottle of dextrose 5% in water and not in another (FDA, 2012).
Again, whereas one drug may be compatible in a solution, a second additive may alter the established pH to such an extent as to make the drugs unstable (Box 18-4). Buffers or antioxidants in a drug may cause two drugs, however, compatible, to precipitate. For
example, ascorbic acid, the buffering component of tetracycline, lowers the pH of the product and therefore may accelerate the decomposition of a drug susceptible to an acid environment.
example, ascorbic acid, the buffering component of tetracycline, lowers the pH of the product and therefore may accelerate the decomposition of a drug susceptible to an acid environment.
BOX 18-4 FACTORS AFFECTING THE STABILITY OF INTRAVENOUS DRUGS AND SOLUTIONS
Precipitation of Parenteral Solution
Some commonly prescribed drugs precipitate when added to IV solutions.
Differences in the physical and chemical properties of each of these solutions may affect the stability of any drug introduced.
A compound soluble in one solution may precipitate in another. Sodium ampicillin deteriorates in acid solutions. This drug, when added to isotonic sodium chloride at a concentration of 30 mg/mL, loses <10% activity in 8 hours. However, when it is added to dextrose 5% in water, usually a more acid solution, and its stability is reduced to a 4-hour period.
pH
A broad pH range (3.5 to 6.5) of dextrose solutions is allowed by the USP. A drug may be stable in one bottle of dextrose 5% in water and not in another.
Additional Drugs
One drug may be compatible in a solution, but a second additive may alter the established pH to such an extent as to make the drugs unstable.
Buffering Agents in Drugs
Presence of buffers or antioxidants, which may cause two drugs, however, compatible, to precipitate. For example, ascorbic acid, the buffering component of tetracycline, lowers the pH of the product and therefore may accelerate the decomposition of a drug susceptible to an acid environment.
Preservatives in the Diluent
Sterile diluents for reconstitution of drugs are available with or without a bacteriostatic agent.
The bacteriostatic agents usually consist of parabens or phenol preservatives.
Certain drugs, including nitrofurantoin, amphotericin B, and erythromycin, are incompatible with these preservatives and should be reconstituted with sterile water for injection.
Degree of Dilution
Solubility often varies with the volume of solution in which a drug is introduced. For example, tetracycline hydrochloride, mixed in a small volume of fluid, maintains its pH range over 24 hours. However, when added to a large volume (1 L), it degrades after 12 hours, becoming less therapeutically active.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access