Pediatric Hematologic Disorders
PEDIATRIC HEMATOLOGIC DISORDERS
Anemia
 Evidence Base
Evidence BaseJanus, J., & Moerschel, S. K. (2010). Evaluation of anemia in children. American Family Physician, 81(12), 1462-1471.
Anemia refers to a deficit of red blood cells (RBCs) or hemoglobin (Hb) in the blood, resulting in decreased oxygen-carrying capacity. It is the most frequent hematologic disorder encountered in children.
Pathophysiology and Etiology
RBCs and Hb are normally formed at the same rate at which they are destroyed. Whenever formation of RBCs or Hb is decreased or their destruction is increased, anemia results. The ability of Hb to carry oxygen to the tissues and remove carbon dioxide for excretion by the lungs is decreased.
May be caused by blood loss related to:
Trauma and ulceration.
Decreased production of platelets.
Increased destruction of platelets.
Decreased number of clotting factors.
May be caused by impairment of RBC production caused by nutritional deficiency.
Iron deficiency—most common type of anemia in ages 6 months to 3 years; approximately 3% of all children.
Folic acid deficiency—causes formation of large RBCs with abnormal nuclear maturation (megaloblastic anemia).
Vitamin B12 deficiency—causes megaloblastic anemia; called pernicious anemia if due to lack of intrinsic factor for absorption of vitamin B12.
Vitamin B6 deficiency.
Lead poisoning—lead absorbed by the bone marrow attaches to newly formed RBCs and inhibits synthesis of heme.
May be caused by decreased erythrocyte production.
Pure RBC anemia.
Secondary hemolytic anemias associated with chronic infection, renal disease, and drugs. In anemia of chronic infection and inflammation, the life span of the RBC is moderately decreased and the ability of the bone marrow to produce RBCs is significantly decreased.
Bone marrow depression—leukemia, aplastic anemias, transient erythrocytopenia of childhood.
May be caused by increased erythrocyte destruction.
Extrinsic factors:
Drugs and chemicals.
Infections—transient erythroblastopenia of childhood caused by parvovirus (fifth disease), usually between ages 6 months and 4 years.
Antibody reactions—passively acquired antibodies against Rh, A, or B isoimmunization, autoimmune hemolytic anemia, burns, poisons (including lead poisoning).
Intrinsic factors:
Abnormalities of the RBC membrane.
Enzymatic defects—glucose-6-phosphate dehydrogenase deficiency.
Abnormal Hb synthesis—sickle cell disease, thalassemia syndromes.
In hemolytic anemias, the RBCs are destroyed at abnormally high rates, primarily by the spleen.
The activity of the bone marrow increases to compensate for the shortened survival time of the RBCs.
Bone marrow hypertrophies and occupies a larger than normal share of the inner structure of bones.
Products of RBC breakdown increase with hemolysis.
Jaundice results when the liver cannot clear the blood of the pigment that results from the breakdown of Hb from destroyed RBCs.
Iron builds up (hemosiderosis) and may deposit on body tissues.
May result from chronic illness such as rheumatoid arthritis and other inflammatory disorders (anemia of chronic disease).
“Physiologic anemia” occurs in term infants at ages 8 to 12 weeks; hematocrit (Hct) should not fall below 30%.
Clinical Manifestations
Condition may be acute or chronic. The more slowly the onset of anemia, the less likely patient will be symptomatic.
Early symptoms:
Listlessness.
Fatigability.
Anorexia related to decreased energy.
Late symptoms:
Pallor.
Weakness.
Tachycardia.
Palpitations.
Tachypnea; shortness of breath on exertion.
Jaundice (with hemolytic anemias).
Diagnostic Evaluation
Complete blood count (CBC) with indices and reticulocytes—vary with types of anemia (see Table 52-1).
Serum iron and total iron-binding capacity—ratio of less than 0.2.
Serum ferritin—less than 12 g/dL.
Lead—greater than 20 g/dL.
Free erythrocyte protoporphyrin—greater than 35 g/dL.
B12, B6, folate levels—may be decreased.
Hb electrophoresis—may show Hb S or other abnormality.
Parvovirus B19 titer—may be elevated in transient erythroblastopenia.
Coombs test.
Table 52-1 Blood Tests in Anemia by Cause | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Management
Iron-Deficiency Anemia
Oral iron at a dose of 3 to 6 mg elemental iron/kg per day given between meals. Reticulocyte count should increase in 7 to 10 days; Hct increases in approximately 4 weeks.
Dietary: decrease milk intake to 16 oz per day; include ironfortified cereals and bread products; increase consumption of red meat; include foods rich in vitamin C.
Currently, iron is rarely administered via intramuscular (I.M.) or intravenous (IV) route because of high incidence of allergic reactions. If administered, monitor the child closely.
Anemia of Chronic Lead Poisoning
See page 1432.
Early detection of high lead levels through screening questionnaires and blood tests.
Maintenance of a well-balanced diet, high in calcium and vitamin D.
Administration of chelating agents such as edetate disodium calcium, dimercaprol, or succimer according to recommendations of the Centers for Disease Control and Prevention.
Use of lead-free paints, gasoline.
Testing of house and soil.
Removal of individuals from unsafe environment.
Megaloblastic Anemia
Folate deficiency—administration of folic acid orally.
B12 deficiency—administration of B12 (cyanocobalamin) I.M.
Transient Erythroblastopenia of Childhood
Spontaneous recovery in 4 to 8 weeks.
For Hb levels of less than 5 g/dL or cardiac failure, transfuse packed RBCs.
Usually, unless cardiac failure occurs, supportive care, not therapy, is provided.
Anemia from Blood Loss or Bone Marrow Suppression
Packed RBC transfusions may be necessary (see page 1004).
Complications
Mental sluggishness, as a result of decreased oxygen and energy for normal neural activity; usually associated with a decreased attention span, decreased intelligence, and lethargy.
Growth retardation related to anorexia and decreased cellular metabolism.
Delayed puberty related to growth retardation.
Cardiac enlargement related to muscle hypertrophy because of increased strain on the heart, attempting to compensate for increased oxygen demand by the tissues; eventually results in heart failure.
Death from cardiac failure related to circulatory collapse and shock.
Nursing Assessment
Obtain history of potential causes.
Dietary history, including the amount of milk and meat consumed; medications.
Family history for genetic causes.
Persistent infection, fever, or chronic disease.
Exposure to drugs, poisons.
Pica—craving and consuming nonfood items (eg, paint chips, paper).
Obtain a baseline assessment.
Observe skin and mucous membranes for pallor.
Obtain height and weight and plot on growth curve.
Measure vital signs, including blood pressure (BP).
Assess child’s functional level—level of exercise tolerance, mental functioning.
Assess attainment of developmental milestones.
Observe for fatigue, listlessness, irritability.
Observe for blood loss: bruising, bleeding, hematuria, or hematochezia (blood in stool). Assess for excessive menstrual bleeding in adolescent females.
 NURSING ALERT
NURSING ALERTBe alert for children at risk for iron-deficiency anemia—children in rapid growth stages (toddlers and adolescents) and pregnant or lactating adolescents.
Nursing Diagnoses
Fatigue related to decreased ability of blood to transport oxygen to the tissues.
Imbalanced Nutrition: Less than Body Requirements of recommended daily dietary allowances.
Risk for Infection related to debilitated state.
Anxiety related to hospitalization and painful diagnostic procedures (venipunctures, fingersticks).
Delayed Growth and Development related to decreased energy.
Nursing Interventions
Minimizing Fatigue
Plan nursing care to allow for lengthy periods when the child is not disturbed by hospital routines, procedures, treatments; set priorities.
Observe for early signs of fatigue, such as irritability, hyperactivity, listlessness.
Encourage sedentary rather than active projects.
Administer oxygen and position upright if dyspnea present.
Do not always encourage self-care.
Provide finger foods that are easy to chew to conserve energy.
Transfuse packed RBCs, as directed.
 NURSING ALERT
NURSING ALERTMonitor transfusion for signs and symptoms of reaction: headache, anxiety, chills, dyspnea, chest pain, hypotension, flank pain, rash, hives, bronchospasm, pruritus, hypertension, more than 1 degree increase in baseline temperature.
Providing Adequate Nutritional Intake
Be aware of the child’s food preferences and plan diet accordingly.
Offer small amounts of food at frequent intervals.
Reward the child for positive attempts to eat.
Allow the child to participate in selection of foods.
Avoid tiring activities and unpleasant procedures at mealtime.
Make mealtime as pleasurable as possible.
Provide iron-rich food and vitamins when necessary.
If iron is ordered, give between meals and with orange juice (iron is absorbed best in an acidic environment).
Limit milk and milk products to 16 to 24 oz per day. Milk products inhibit the absorption of oral iron.
Administer liquid iron with a dropper or straw or dilute with water or fruit juice to prevent staining the teeth.
If administered by dropper, make sure that liquid iron is deposited in the back of the mouth.
Dental stains can be removed by brushing the teeth with sodium bicarbonate or hydrogen peroxide and then rinsing with water after each administration.
Be alert for adverse effects of iron supplements—gastric distress, colicky pain, diarrhea, or constipation; may call for decreased dose.
Advise family that child’s stool may turn dark green or black.
Stress the importance of continuing iron therapy according to health care provider’s directions, even though the child may not appear to be ill.
Inform parents that iron overdoses can be harmful or fatal.
Preventing Infection
Reducing Anxiety
Allow the child to handle equipment used for tests and procedures (tourniquets, syringes).
Explain all procedures and the treatment plan to the child in a way that the child can understand.
Allow the older child to look through a microscope at a blood smear, if interested and if appropriate for child’s age.
Permit the child to cleanse the area for a venipuncture or a fingerstick and to choose the finger.
Promoting Normal Growth and Development
Make sure that nutrition is adequate for age and activity level.
Encourage participation in age-related activities.
Encourage doing homework and tutored activities.
Encourage peer socialization.
Promote age-appropriate play and therapeutic play.
Perform periodic growth chart evaluation and developmental testing.
Share results with parents and explain the association between diet/anemia and growth and development.
Notify health care provider and make referrals, as indicated.
Family Education and Health Maintenance
Stress to the parents the importance of continuing the iron therapy according to the provider’s directions even though the child may not appear ill.
Initiate and reinforce good dietary habits.
Foods rich in iron include dark green leafy vegetables, fortified cereals, dried fruits, nuts, and red meats.
Do not allow the child to drink excessive quantities of milk to the exclusion of other foods that contain more iron. Limit milk intake to 16 to 24 oz per day.
Provide vitamin supplements, if necessary. Vitamin C appears to enhance the absorption of iron.
Explain the reasons for diet change to parents in language they can understand. Visual aids and pictures may be helpful.
Assist the parents to select iron-rich foods that are acceptable to the child, within the family’s food budget, and culturally acceptable.
Initiate a nutritional consultation, as indicated.
Discuss with parents any of the social, economic, and environmental problems that may contribute to the child’s disease.
Emphasize to the parents the benefits of a referral to a community health nurse if it appears that the family will need support in dealing with the child’s chronic disease.
Discuss general health measures, including adequate rest, diet, sunshine, and fresh-air activity.
Encourage regular medical and dental evaluations. Emphasize the need for appropriate follow-up visits.
Explain that infection may be prevented by dressing the child according to the weather and by keeping the child away from people with colds, sore throats, and other infections.
Teach the parents how to administer medication.
Alert the parents to signs of disease progression—increased fatigue, pallor, weakness, developmental delays, and poor performance in school and activities.
Prepare for possible adverse GI effects, including constipation and change in stool color.
 DRUG ALERT
DRUG ALERTMuch variation exists in the elemental iron content of commercially available liquid preparations that contain iron. To avoid confusion, the dosage should be expressed in terms of elemental iron and then converted to the proper amount of the therapeutic agent selected.
Evaluation: Expected Outcomes
Increasing activity noted.
Eats frequent small feedings of cereal, bread, red meat, vegetables; tolerates iron supplement without adverse effects.
Remains free from infection; normal temperature.
Cooperates with frequent blood sampling.
Maintains growth curve; manages age-appropriate developmental activities.
Sickle Cell Disease (Sickle Cell Anemia)
 Evidence Base
Evidence BaseMcCavit, T. L. (2011). Sickle cell disease. Pediatrics in Review, 33, 195-206.
Sickle cell disease is a severe, chronic, hemolytic anemia occurring in people who possess two copies of the sickle gene (homozygous). The clinical course is characterized by episodes of pain caused by the occlusion of small blood vessels by sickled RBCs. Persons heterozygous (with one copy) for the sickling gene are said to possess the sickle cell trait, which is associated with a benign clinical course. Sickle cell disease is found almost entirely in blacks and people of Arabic or northern Mediterranean ancestry. Approximately 8% of blacks have the sickle cell trait. Approximately 1 of every 500 blacks and 1 of every 1,000 to 1,400 Hispanic American infants born in the United States has sickle cell anemia. Approximately 90,000 people in the United States are affected.
Pathophysiology and Etiology
Genetically determined, inherited disease—autosomal recessive.
Each person inherits one gene from each parent, which governs the synthesis of Hb (see Table 52-2).
Normally, each Hb molecule consists of four molecules of heme folded into one molecule of globin. Each globin molecule consists of two alpha chains and two beta chains.
The amino acid sequence on the chain is altered in sickle cell Hb—valine is substituted for glutamic acid in the sixth position of the 574 amino acids that make up the globin fraction of Hb.
Sickle cell Hb aggregates into elongated crystals under conditions of low oxygen concentration, acidosis, and dehydration.
This distorts the membrane of the RBC, causing it to assume a crescent or sickle shape. The cells easily become entangled and enmeshed, leading to increased blood viscosity, vessel occlusion, and tissue necrosis.
Sickled RBCs are fragile and are rapidly destroyed in the circulation; they live 6 to 20 days versus 120 days for normal RBCs.
Anemia results when the rate of destruction of RBCs is greater than the rate of production.
Increased sequestration of RBCs occurs in the spleen.
Table 52-2 Transmission of Sickle Cell Disease | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Clinical Manifestations
Children are rarely symptomatic until late in the first year of life, related to increased amounts of fetal hemoglobin (Hb F). Clinical manifestations are sporadic; the child may be asymptomatic for several months. Periods of crisis occur at variable intervals.
Signs of Anemia
May last 1 to 2 weeks and subside spontaneously.
Hb level—6 to 9 g/dL.
Loss of appetite.
Paleness.
Weakness.
Fever.
Irritability.
Jaundice; increased hemolysis results in hemosiderosis (increased iron storage in the liver).
Precipitating Factors of Crisis
Dehydration.
Infection.
Trauma.
Strenuous physical exertion.
Extreme fatigue.
Cold exposure.
Hypoxia.
Acidosis.
Surgery.
Pregnancy.
Sickle Cell (Vaso-occlusive) Crisis
Most common form of crisis.
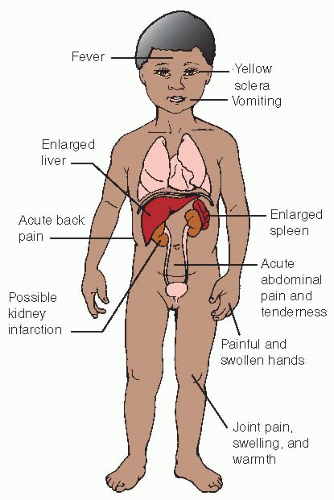
Small blood vessels are occluded by the sickle-shaped cells, causing distal ischemia and infarction (see Figure 52-1).
Extremities:
Bony destruction—related to erythroid hyperplasia of marrow, leading to osteoporosis or ischemic necrosis.
Bone pain; painful and swollen large joints.
Dactylitis (“hand-foot” syndrome)—aseptic infarction of metacarpals and metatarsals, causing symmetrical swelling and pain; commonly first vaso-occlusive event seen in infants and toddlers.
Spleen:
Abdominal pain.
Splenomegaly—initially enlarges because of increased activity at site of RBC hemolysis; increased size results in discomfort.
After multiple episodes of splenic vaso-occlusion, the spleen becomes fibrotic and atrophied.
Decreased splenic function increases the risk of infection, especially by encapsulated organisms such as Streptococcus pneumoniae.
Cerebral occlusion:
Stroke.
Hemiplegia.
Retinal damage, leading to blindness.
Seizures.
Pulmonary infarction.
Altered renal function: enuresis, hematuria.
Impaired liver function.
Priapism—abnormal, recurrent, prolonged, painful penile erection.
Splenic Sequestration Crisis
Large amounts of blood become pooled in the spleen.
Spleen becomes massively enlarged.
Great decrease in RBC mass occurs within hours.
Signs of circulatory collapse (eg, shock and hypotension) develop rapidly.
Frequent cause of death in infants with sickle cell disease.
Exchange blood transfusion may be required.
Aplastic Crisis
Bone marrow ceases production of RBCs only.
Results in low reticulocyte counts.
Chronic Symptoms
Chronic organ damage results in organ dysfunction.
Jaundice.
Gallstones.
Progressive impairment of kidney function.
Fibrotic spleen, resulting in high susceptibility to Haemophilus influenzae and S. pneumoniae infections, osteomyelitis, and pneumococcal septicemia.
Growth retardation of the long bones and spine deformities.
Delayed growth and puberty.
Cardiac decompensation related to chronic anemia (may develop heart failure).
Chronic, painful leg ulcers related to decreased peripheral circulation and unrelated to injury; may take months to heal or may not heal without intense therapy, including blood transfusions and grafting.
Decreased life span.
Altered bony structures—aseptic necrosis of the bones, especially the femoral and humoral heads.
Diagnostic Evaluation
Sickle cell prep (sickling test):
Done by finger or heelstick.
Oxygen is removed from a drop of blood.
The blood is observed under the microscope for sickle-shaped cells.
Test does not distinguish between people with sickle cell trait and disease or other sickle hemoglobinopathies.
Routinely done on infants shortly after birth.
Sickledex:
Done by fingerstick.
A small amount of blood is placed in a solution containing a chemical-reducing agent.
Sickle hemoglobin is indicated if the solution turns cloudy.
Test also does not distinguish between people with sickle cell trait and disease or other sickle hemoglobinopathies.
Hemoglobin electrophoresis:
Requires venipuncture.
Hb is subjected to an electric current that separates the various types and determines the amounts present.
Test is used to diagnose both sickle cell trait and sickle cell disease if two types of Hb are demonstrated in approximately equal amounts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access