Neurologic and sensory care
A neurologic examination provides a record of vital information regarding the patient’s neurologic function. Its purpose is to determine the presence of nervous system dysfunction. A skilled examiner knows the proper technique for testing function and is familiar with the expected normal responses to testing. Effective neurologic care aims to preserve and restore optimal nervous system function. Precise nursing skills and meticulous attention to detail are indispensable to achieving effective care. It’s also important that you’re aware of the standards of care endorsed by the American Academy of Neurology AAN, the American Association of Neuroscience Nurses AANN, the American Association of Neurological Surgeons AANS, the American Heart Association AHA, the American Pain Society APS, the Infusion Nurses Society INS, and the National Institute of Neurological Disorders and Stroke NINDS. In addition to being supported by evidence-based EB data and fundamental principles of science SCIENCE, the best practices presented in this chapter are supported by guidelines of the Centers for Disease Control and Prevention CDC, the National Institutes of Health NIH, the Joint Commission on Accreditation of Healthcare Organizations JCAHO, and the tenets of the American Hospital Association’s Patient Care Partnership PCP. For points on equipment, manufacturers MFR may recommend specific guidelines. Together, they’ll provide you with the information necessary to care for patients with neurologic deficits or injury.
Neurologic assessment and examination
A neurologic physical examination may be conducted by the physician or by an advanced practice nurse. For nursing purposes, this examination is used to determine whether nervous system dysfunction is present and to determine the patient’s responses to actual or potential health problems precipitated by the dysfunction. The neurologic examination is typically preceded by a physical examination and history. It should be conducted in a systematic, hierarchical approach from the highest level of function (cerebral cortex) to the lowest (reflexes) and should include a review of mental state, cranial nerve, motor and sensory systems, and cerebellar function and reflexes.
The first step in a neurologic examination is to assess neurologic vital signs, starting with the patient’s level of consciousness and orientation level. Many patients with neurologic disorders experience changes in perception — from confusion to psychosis — from neurologic dysfunction. Unaddressed, such disorientation further impairs the patient’s ability to participate in recovery. Recognizing this will help you intervene properly.
To record or track assessment findings, use special flowcharts or neurologic assessment forms. In common use at most health care facilities, these charts and forms separate and grade components of a neurologic assessment, assisting the nurse and other caregivers to quickly recognize changes in neurologic status and to plan subsequent patient care.
Respiratory assessment constitutes an important part of an overall neurologic assessment because patients with neurologic damage — especially those with traumatic brain or spinal cord injuries — are at considerable risk for respiratory complications. Such injuries may depress the respiratory control center and paralyze the muscles used for breathing. As a result, brain tissue, which is especially sensitive to blood oxygen levels, can quickly be damaged by inadequate oxygenation.
Thorough respiratory care goes hand in hand with neurologic care. Frequent position changes, chest physiotherapy, and tracheal suctioning are typical interventions. Additional techniques for preventing complications and promoting comfort include pain management and maintaining a quiet, stress-free environment.
Rehabilitation
Neurologic rehabilitation begins on admission and touches all aspects of daily care. Because neurologic impairment can alter every area of function, the patient’s identity may change. Consequently, rehabilitation procedures must address the psychosocial and physiologic changes associated with the patient’s condition — a task that requires enormous time and patience.
The success of rehabilitation efforts may hinge largely on a patient’s ability to adapt to significant — even profound — changes. A few of the factors that influence this ability to adapt include the patient’s age, the deficit itself, and available support systems. Another ingredient needed for effective rehabilitation is sensitive and skilled nursing care. Such care can dramatically improve the patient’s prospects for positive adaptation and recovery.
Cerebrospinal fluid drains
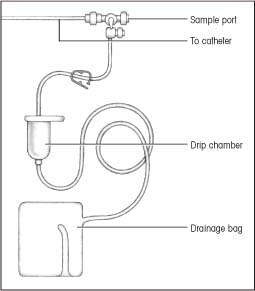
Cerebrospinal fluid (CSF) drainage aims to reduce CSF pressure to the desired level and then to maintain it at that level. Fluid is withdrawn from the lateral ventricle (ventriculostomy). Ventricular drainage is used to reduce increased intracranial pressure (ICP). External CSF drainage is used most commonly to manage increased ICP and to facilitate spinal or cerebral dural healing after traumatic injury or surgery. In either case, CSF is drained by a catheter or a ventriculostomy tube in a sterile, closed drainage collection system.
Other therapeutic uses include ICP monitoring via the ventriculostomy, direct instillation of medications, contrast media, or air for diagnostic radiology, and aspiration of CSF for laboratory analysis.
To place the ventricular drain, the physician inserts a ventricular catheter through a burr hole in the patient’s skull. Usually, this is done in the operating room, with the patient receiving a general anesthetic. (See CSF drainage, page 368.)
Equipment
Overbed table • sterile gloves • sterile cotton-tipped applicators • chlorhexidine solution • alcohol pads • sterile fenestrated drape • 3-ml syringe for local anesthetic • 25G ¾″ needle for injecting anesthetic • local anesthetic (usually 1% lidocaine [Xylocaine]) • 18G or 20G sterile spinal needle or Tuohy needle • #5 French whistle-tip catheter or ventriculostomy tube • external drainage set (includes drainage tubing and sterile collection bag) • suture material • 4″ × 4″ dressings • paper tape • lamp or another light source • I.V. pole • ventriculostomy tray and twist drill • sterile marker • sterile labels • optional: pain medication (such as an analgesic) and antiinfective agent (such as an antibiotic)
Preparation of equipment
Open all equipment using sterile technique.
Check all packaging for breaks in seals and for expiration dates.
After the physician places the catheter, connect it to the external drainage system tubing.
Secure connection points with tape or a connector.
Place the collection system, including drip chamber and collection bag, on an I.V. pole.
Implementation
Explain the procedure to the patient and his family. Consent should be obtained by the physician from the patient or a responsible family member and should be documented according to your facility’s policy. PCP
Perform a baseline neurologic assessment, including vital signs, to help detect alterations or signs of deterioration. AANN
Wash your hands thoroughly. CDC
Inserting a ventricular drain
Place the patient in a supine position.
Place the equipment tray on the overbed table, and unwrap the tray. Label all medications, medication containers, and other solutions on and off the sterile field. JCAHO
Adjust the height of the bed so that the physician can perform the procedure comfortably.
Illuminate the area of the catheter insertion site.
The physician will clean the insertion site and administer a local anesthetic. He’ll put on sterile gloves and drape the insertion site.
To insert the drain, the physician will request a ventriculostomy tray with a twist drill. After completing the ventriculostomy, he’ll connect the drainage system and suture the ventriculostomy in place. He’ll then cover the insertion site with a sterile dressing.
CSF drainage
Cerebrospinal fluid (CSF) drainage aims to control intracranial pressure (ICP) during treatment for traumatic injury or other conditions that cause a rise in ICP. A ventricular drain using a closed drainage system is detailed below.
Ventricular drain
For a ventricular drain, the physician makes a burr hole in the patient’s skull and inserts the catheter into the ventricle. The distal end of the catheter is connected to a closed drainage system.
Monitoring CSF drainage
Maintain a continuous hourly output of CSF. Ensure that the flow chamber of the ICP monitoring setup remains positioned as ordered. AANN NIH
To drain CSF as ordered, put on sterile gloves, and then turn the main stopcock on to drainage. This allows CSF to collect in the graduated flow chamber. Document the time and the amount of CSF obtained. Turn the stopcock off to drainage. To drain the CSF from this chamber into the drainage bag, release the clamp below the flow chamber. Never empty the drainage bag. Instead, replace it when full using sterile technique. AANN CDC NIH
Check the dressing frequently for drainage, which could indicate CSF leakage. AANN NIH
Check the tubing for patency by watching the CSF drops in the drip chamber.
Observe CSF for color, clarity, amount, blood, and sediment. CSF specimens for laboratory analysis should be obtained from the collection port attached to the tubing, not from the collection bag.
Change the collection bag when it’s full or every 24 hours, according to your facility’s policy. AANN
Special considerations
Maintenance of a continual hourly output of CSF is essential to prevent overdrainage or underdrainage. Underdrainage or lack of CSF may reflect kinked tubing, catheter displacement, or a drip chamber placed higher than the catheter insertion site. Overdrainage can occur if the drip chamber is placed too far below the catheter insertion site.
Raising or lowering the head of the bed can affect the CSF flow rate. When changing the patient’s position, reset the system to zero. AANN
If the patient is ambulatory or is allowed out of bed, advise him that he must call for assistance before getting out of bed. The drain must be closed before getting the patient out of bed.
The patient may experience chronic headache during continuous CSF drainage. Reassure him that this isn’t unusual; administer analgesics as appropriate. AANN
For ventricular drains, make sure ICP waveforms are being monitored at all times. AANN
Nursing diagnoses
Decreased intracranial adaptive capacity
Risk for infection
Expected outcomes
The patient will:
maintain ICP within normal limits
show no evidence of neurologic compromise
remain free from infection.
Treatment and adverse effects of ICP control
Listed below are the common medications and nursing interventions used to decrease elevated (greater than 20 mm Hg) intracranial pressure (ICP) and the adverse effects of each treatment.
| Treatment | Application | Adverse effects |
|---|---|---|
| Barbiturates | To induce barbiturate coma as a last resort | Hypotension, loss of neurologic examination, cardiac abnormalities |
| Hyperventilation | To reduce carbon dioxide and dilate cerebral vasculature to lower ICP | Reduced cerebral blood flow and oxygenation |
| Hypothermia | To possibly benefit moderately severe head injuries | Cardiac suppression, renal dysfunction |
| Mannitol (Osmitrol) | To reduce acute ICP elevation; given as an I.V. bolus | Dehydration, renal failure |
| Neuromuscular paralysis | To reduce activity that could elevate ICP; given in conjunction with sedatives | Respiratory compromise |
| Sedation | To reduce elevated breakthrough ICP in conjunction with paralysis | Hypotension |
Complications
Signs of excessive CSF drainage include headache, tachycardia, diaphoresis, and nausea.
Acute overdrainage may result in collapsed ventricles, tonsillar herniation, and medullary compression.
Cessation of drainage may indicate clot formation. If you can’t quickly identify the cause of the obstruction, notify the physician. If drainage is blocked, the patient may develop signs of increased ICP.
Infection may cause meningitis. To prevent this, administer antibiotics as ordered.
Documentation
Record the time and date of the insertion procedure and the patient’s response. Record routine vital signs and neurologic assessment findings at least every 4 hours.
Document the color, clarity, and amount of CSF at least every 8 hours. Record hourly and 24-hour CSF output, and describe the condition of the dressing.
Supportive references
American Association of Neuroscience Nurses. Core Curriculum for Neuroscience Nursing, 4th ed. Philadelphia: W.B. Saunders Co., 2004.
Barker, E. Neuroscience Nursing, 2nd ed. St. Louis: Mosby–Year Book, Inc., 2002.
Hickey, J.V. The Clinical Practice of Neurological and Neurosurgical Nursing, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2003.
Lynn-McHale Wiegand, D.J., and Carlson, K.K. (eds.) AACN Procedure Manual for Critical Care, 5th ed. Philadelphia: W.B. Saunders Co., 2005.
Intracranial pressure monitoring
Intracranial pressure (ICP) monitoring measures pressure exerted by the brain, blood, and cerebrospinal fluid (CSF) against the inside of the skull. Normal ICP is 0 to 15 mm Hg, with the ICP threshold of 20 to 25 mm Hg as the highest acceptable limit before instituting treatment. AANS Indications for monitoring ICP include head trauma with bleeding or edema, overproduction or insufficient absorption of CSF, cerebral hemorrhage, and space-occupying brain lesions. ICP monitoring can detect elevated ICP early,
before clinical danger signs develop. Prompt intervention can then help avert or diminish neurologic damage caused by cerebral hypoxia and shifts of brain mass. (SeeTreatment and adverse effects of ICP control, page 369.)
before clinical danger signs develop. Prompt intervention can then help avert or diminish neurologic damage caused by cerebral hypoxia and shifts of brain mass. (SeeTreatment and adverse effects of ICP control, page 369.)
Understanding ICP monitoring
Intracranial pressure (ICP) can be monitored using one of four systems.
Intraventricular catheter monitoring
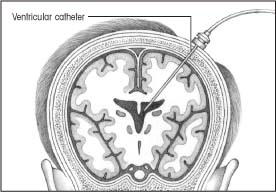 |
In intraventricular catheter monitoring, which monitors ICP directly, the physician inserts a small polyethylene or silicone rubber catheter into the lateral ventricle through a burr hole.
Although this method measures ICP most accurately, it carries the greatest risk of infection. This is the only type of ICP monitoring that allows evaluation of brain compliance and drainage of significant amounts of cerebrospinal fluid (CSF).
Contraindications usually include stenotic cerebral ventricles, cerebral aneurysms in the path of catheter placement, and suspected vascular lesions.
Subarachnoid bolt monitoring
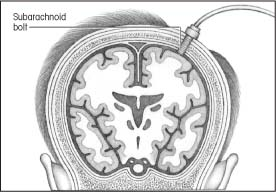 |
Subarachnoid bolt monitoring involves insertion of a special bolt into the subarachnoid space through a twist-drill burr hole that’s positioned in the front of the skull behind the hairline.
Placing the bolt is easier than placing an intraventricular catheter, especially if a computed tomography scan reveals that the cerebrum has shifted or the ventricles have collapsed. This type of ICP monitoring also carries less risk of infection and parenchymal damage because the bolt doesn’t penetrate the cerebrum.
Epidural or subdural sensor monitoring
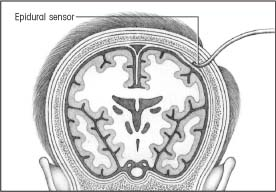 |
ICP can also be monitored from the epidural or subdural space. For epidural monitoring, a fiber-optic sensor is inserted into the epidural space through a burr hole. This system’s main drawback is its questionable accuracy because ICP isn’t being measured directly from a CSF-filled space.
For subdural monitoring, a fiber-optic transducer-tipped catheter is tunneled through a burr hole, and its tip is placed on brain tissue under the dura mater. The main drawback to this method is its inability to drain CSF.
Intraparenchymal monitoring
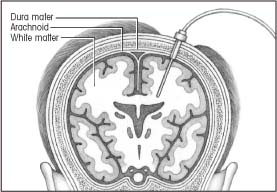 |
In intraparenchymal monitoring, the physician inserts a catheter through a small subarachnoid bolt and, after puncturing the dura, advances the catheter a few centimeters into the brain’s white matter. There’s no need to balance or calibrate the equipment after insertion.
Although this method doesn’t provide direct access to CSF, measurements are accurate because brain tissue pressure correlates well with ventricular pressures. Intraparenchymal monitoring may be used to obtain ICP measurements in patients with compressed or dislocated ventricles.
The four basic ICP monitoring systems are intraventricular catheter, subarachnoid bolt, epidural sensor, and intraparenchymal pressure monitoring. (See Understanding ICP monitoring.)
Regardless of which system is used, the procedure is typically performed by a neurosurgeon in the operating room, emergency department, or intensive care unit. Insertion of an ICP monitoring device requires sterile technique to reduce the risk of central nervous system (CNS) infection. Setting up equipment for the monitoring systems also requires strict asepsis. AANN AANS
Equipment
Monitoring unit and transducers as ordered • 16 to 20 sterile 4″ × 4″ gauze pads • linen-saver pads • shave preparation tray or hair scissors • sterile drapes • chlorhexidine solution • sterile gown • surgical mask • sterile gloves • head dressing supplies (two rolls of 4″ elastic gauze dressing, one roll of 4″ roller gauze, adhesive tape) • sterile marker • sterile labels • optional: suction apparatus, I.V. pole, and yardstick
Preparation of equipment
Monitoring units and setup protocols are varied and complex and differ among health care facilities. Check your facility’s guidelines for your particular unit.
Various types of preassembled ICP monitoring units are available, each with its own setup protocols.
These units are designed to reduce the risk of infection by eliminating the need for multiple stopcocks, manometers, and transducer dome assemblies. Some facilities use units that have miniaturized transducers rather than transducer domes.
Implementation
Explain the procedure to the patient or his family. Make sure the patient or a responsible family member has signed a consent form. PCP
Determine whether the patient is allergic to iodine preparations.
Provide privacy if the procedure is being done in an open emergency department or intensive care unit. PCP
Wash your hands. CDC
Obtain baseline routine and neurologic vital signs to aid in prompt detection of decompensation during the procedure. AANN
Place the patient in the supine position, and elevate the head of the bed 30 degrees (or as ordered). AANN
Place linen-saver pads under the patient’s head. Clip his hair at the insertion site, as indicated by the physician, to decrease the risk of infection. Carefully fold and remove the linen-saver pads to avoid spilling loose hair onto the bed. Drape the patient with sterile drapes. Scrub the insertion site for 2 minutes with chlorhexidine solution. CDC
Setting up an ICP monitoring system
To set up an intracranial pressure (ICP) monitoring system, follow these steps, using strict sterile technique. CDC
Begin by opening a sterile towel. On the sterile field, place a 20-ml luer-lock syringe, an 18G needle, a 250-ml bag filled with normal saline solution (with outer wrapper removed), and a disposable transducer.
Put on sterile gloves and gown, and fill the 20-ml syringe with normal saline solution from the I.V. bag. CDC
Remove the injection cap from the patient line and attach the syringe. Turn the system stopcock off to the short end of the patient line, and flush through to the drip chamber (as shown). Allow a few drops to flow through the flow chamber (the manometer), the tubing, and the one-way valve into the drainage bag. (Fill the tubing and the manometer slowly to minimize air bubbles. If any air bubbles surface, be sure to force them from the system.)
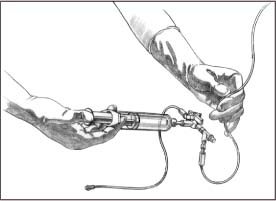 |
Attach the manometer to the I.V. pole at the head of the bed.
Slide the drip chamber onto the manometer, and align the chamber to the zero point (as shown).
Next, connect the transducer to the monitor.
Put on a clean pair of sterile gloves. CDC
Keeping one hand sterile, turn the patient stopcock off to the patient.
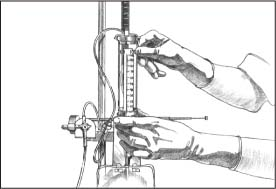 |
Align the zero point with the center line of the patient’s head, level with the middle of the ear (as shown).
Lower the flow chamber to zero, and turn the stopcock off to the dead-end cap. With a clean hand, balance the system according to monitor guidelines.
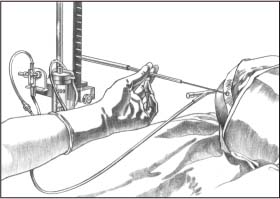 |
Turn the system stopcock off to drainage, and raise the flow chamber to the ordered height (as shown).
Return the stopcock to the ordered position, and observe the monitor for the return of ICP patterns.
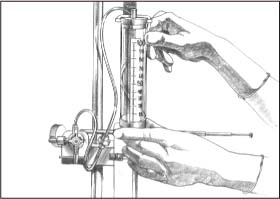 |
To facilitate placement of the device, hold the patient’s head in your hands or attach a long strip of 49 roller gauze to one side rail, and bring it across the patient’s forehead to the opposite rail. Reassure the conscious patient to help ease his anxiety. Talk to him frequently to assess his level of consciousness (LOC) and detect signs of deterioration.Watch for cardiac arrhythmias and abnormal respiratory patterns.
After insertion, put on sterile gloves and apply chlorhexidine solution and a sterile dressing to the site. If not done by the physician, connect the catheter to the appropriate monitoring device, depending on the system used. CDC (SeeSetting up an ICP monitoring system.)
If the physician has set up a ventriculostomy drainage system, attach the drip chamber to the headboard or bedside I.V. pole as ordered.
Inspect the insertion site at least every 24 hours (or according to your facility’s policy) for redness, swelling, and drainage. Clean the site, reapply chlorhexidine solution, and apply a fresh sterile dressing.
Assess the patient’s clinical status, and take routine and neurologic vital signs every hour, or as ordered.
Make sure you’ve obtained orders for waveforms and pressure parameters from the physician.
Calculate cerebral perfusion pressure (CPP) hourly; use the equation: CPP = MAP − ICP (MAP refers to mean arterial pressure).
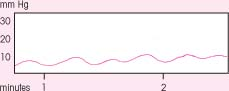
Observe digital ICP readings and waves. Remember, the pattern of readings is more significant than any single reading. (See Interpreting ICP waveforms, page 374.) If you observe continually elevated ICP readings, note how long they’re sustained. If they last several minutes, notify the physician immediately. Finally, record and describe any CSF drainage.
Special considerations
Osmotic diuretic agents such as mannitol (Osmitrol) reduce cerebral edema by shrinking intracranial
contents. Given by I.V. drip or bolus, mannitol draws water from tissues into plasma; it doesn’t cross the blood-brain barrier. Monitor serum electrolyte levels and osmolality readings closely because the patient may become dehydrated very quickly. Be aware that a rebound increase in ICP may occur. (See Managing increased ICP.) SCIENCE
Interpreting ICP waveforms
Three waveforms — A, B, and C — are used to monitor intracranial pressure (ICP). A (plateau) waves are an ominous sign of intracranial decompensation and poor compliance. B waves correlate with changes in respiration, and C waves correlate with changes in arterial pressure.
Normal waveform
A normal ICP waveform typically shows a steep upward systolic slope followed by a downward diastolic slope with a dicrotic notch. In most cases, this waveform occurs continuously and indicates an ICP between 0 and 15 mm Hg — normal pressure.
 |
A waves
The most clinically significant ICP waveforms are A waves (shown at right), which may reach elevations of 50 to 100 mm Hg, persist for 5 to 20 minutes, then drop sharply — signaling exhaustion of the brain’s compliance mechanisms. A waves may come and go, spiking from temporary rises in thoracic pressure or from any condition that increases ICP beyond the brain’s compliance limits. Activities, such as sustained coughing or straining during defecation, can cause temporary elevations in thoracic pressure.
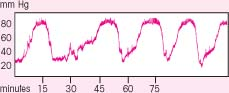 |
B waves
B waves, which appear sharp and rhythmic with a sawtooth pattern, occur every 1½ to 2 minutes and may reach elevations of 50 mm Hg. The clinical significance of B waves isn’t clear, but the waves correlate with respiratory changes and may occur more frequently with decreasing compensation. Because B waves sometimes precede A waves, notify the physician if B waves occur frequently.
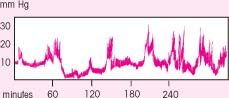 |
C waves
Like B waves, C waves are rapid and rhythmic, but they aren’t as sharp. Clinically insignificant, they may fluctuate with respirations or systemic blood pressure changes.
 |
Managing increased ICP
By performing nursing care gently, slowly, and cautiously, you can best help manage — or even significantly reduce — increased intracranial pressure (ICP). If possible, urge your patient to participate in his own care. Here are some steps you can take to manage increased ICP.
Plan your care to include rest periods between activities. This allows the patient’s ICP to return to baseline, thus avoiding lengthy and cumulative pressure elevations.
Speak to the patient before attempting any procedures, even if he appears comatose. Touch him on an arm or leg first before touching him in a more personal area, such as the face or chest. This is especially important if the patient doesn’t know you or if he’s confused or sedated.
Suction the patient 10 seconds or less and only when needed to remove secretions and maintain airway patency. Avoid depriving him of oxygen for long periods while suctioning; always hyperventilate the patient with oxygen before and after the procedure. Monitor his heart rate while suctioning. If multiple catheter passes are needed to clear secretions, hyperventilate the patient between them to bring ICP as close to baseline as possible.
To promote venous drainage, keep the patient’s head in the midline position, even when he’s positioned on his side. Avoid flexing the neck or hip more than 90 degrees, and keep the head of the bed elevated 30 to 45 degrees.
To avoid increasing intrathoracic pressure, which raises ICP, discourage Valsalva’s maneuver and isometric muscle contractions. To avoid isometric contractions, distract the patient when giving him painful injections (by asking him to wiggle his toes and by massaging the area before injection to relax the muscle) and have him concentrate on breathing through difficult procedures such as bed-to-stretcher transfers. To keep the patient from holding his breath when moving around in bed, tell him to relax as much as possible during position changes. If necessary, administer a stool softener to help prevent constipation and unnecessary straining during defecation.
If the patient is heavily sedated, monitor his respiratory rate and blood gas levels. Depressed respirations will compromise ventilations and oxygen exchange. Maintaining adequate respiratory rate and volume helps reduce ICP.
If you’re in a specialty unit, you may be able to routinely hyperventilate the patient to counter sustained ICP elevations. This procedure is one of the best ways to reduce high ICP at bedside for short periods. Consult your facility’s protocol.
To avoid rebound increased ICP, 50 ml of albumin may be given with the mannitol bolus. Note, however, that you’ll see a residual rise in ICP before it decreases. If your patient has heart failure or severe renal dysfunction, monitor for problems in adapting to the increased intravascular volumes.
Fluid restriction, usually 1,200 to 1,500 ml/day, prevents cerebral edema from developing or worsening.
Barbiturate-induced coma depresses the reticular activating system and reduces the brain’s metabolic demand. Reduced demand for oxygen and energy reduces cerebral blood flow, thereby lowering ICP. EB SCIENCE
Hyperventilation with oxygen from a handheld resuscitation bag or ventilator helps rid the patient of excess carbon dioxide, thereby constricting cerebral vessels and reducing cerebral blood volume and ICP. However, only normal brain tissues respond because blood vessels in damaged areas have reduced vasoconstrictive ability. SCIENCE
Because fever raises brain metabolism, which increases cerebral blood flow, fever reduction (achieved by administering acetaminophen [Tylenol], sponge baths, or a hypothermia blanket) also helps to reduce ICP. However, rebound increases in ICP and brain edema may occur if rapid rewarming takes place after hypothermia or if cooling measures induce shivering.
Withdrawal of CSF through the drainage system reduces CSF volume and thus reduces ICP. Although less commonly used, surgical removal of a skull-bone flap provides room for the swollen brain to expand. If this procedure is performed, keep the site clean and dry to prevent infection and maintain sterile technique when changing the dressing. AANN
Nursing diagnoses
Decreased intracranial adaptive capacity
Risk for infection
Expected outcomes
The patient will:
maintain ICP within normal limits
show no evidence of neurologic compromise
remain free from infection.
Complications
CNS infection, the most common hazard of ICP monitoring, can result from contamination of the equipment setup or of the insertion site.
Watch for signs of impending or overt decompensation: pupillary dilation (unilateral or bilateral), decreased pupillary response to light, decreasing LOC, rising systolic blood pressure and widening pulse pressure, bradycardia, slowed, irregular respirations and, in late decompensation, decerebrate posturing.
Documentation
Record the time and date of the insertion procedure and the patient’s response. Note the insertion site and the type of monitoring system used. Record ICP digital readings and waveforms and CPP hourly in your notes, on a flowchart, or directly on readout strips, depending on your facility’s policy. Document any factors that may affect ICP (for example, drug therapy, stressful procedures, or sleep).
Record routine and neurologic vital signs hourly, and describe the patient’s clinical status. Note the amount, character, and frequency of any CSF drainage (for example, “between 6 p.m. and 7 p.m., 15 ml of blood-tinged CSF”). Record the ICP reading in response to drainage.
Supportive references
American Association of Neuroscience Nurses. Core Curriculum for Neuroscience Nursing, 4th ed. Philadelphia: W.B. Saunders Co., 2004.
Barker, E. Neuroscience Nursing, 2nd ed. St. Louis: Mosby–Year Book, Inc., 2002.
Hickey, J.V. The Clinical Practice of Neurological and Neurosurgical Nursing, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2003.
Kirkness, C.J., et al. “Intracranial Pressure Waveform Analysis: Clinical and Research Implications”, Journal of Neuroscience Nursing 32(5):271-77, October 2000.
Lynn-McHale Wiegand, D.J., and Carlson, K.K. (eds.) AACN Procedure Manual for Critical Care, 5th ed. Philadelphia: W.B. Saunders Co., 2005.
March, K. “Intracranial Pressure Monitoring and Assessing Intracranial Compliance in Brain Injury”, Critical Care Nursing Clinics of North America 12(4):429-35, December 2000.
Morton, P.G., et al. Critical Care Nursing: A Holistic Aproach, 8th ed. Philadelphia: Lippincott Williams & Wilkins, 2005. EB
Jugular venous oxygen saturation monitoring
Jugular venous oxygen saturation (SjvO2) monitoring measures the venous oxygenation saturation of blood as it leaves the brain. It reflects the oxygen saturation of blood after cerebral perfusion has taken place. After comparing SjvO2 with the arterial venous oxygenation, you can determine if blood flow to the brain matches the brain’s metabolic demand.
SjvO2 monitoring is often used with other types of cerebral hemodynamic monitoring, such as intracranial
pressure (ICP) monitoring, to provide better information about pressure and perfusion during treatment. Treatment regimens can be titrated to enhance pressure and perfusion.
pressure (ICP) monitoring, to provide better information about pressure and perfusion during treatment. Treatment regimens can be titrated to enhance pressure and perfusion.
The normal range for SjvO2 is 55% to 70%. Values higher than 70% indicate hyperperfusion. Values between 40% and 54% indicate relative hypoperfusion. Values lower than 40% indicate ischemia.
Data from monitoring can also be used to calculate cerebral extraction of oxygen (CeO2 = oxygen saturation in arterial blood [SaO2] − SjvO2), cerebral arterial oxygen content (CaO2 = 1.34 × hemoglobin [Hb] × SaO2 − 0.0031 × partial pressure of arterial oxygen [PaO2]), the global cerebral content saturation (CjvO2 = 1.34 × Hb × SjvO2 + 0.0031 × PjvO2), arteriovenous jugular oxygen content (AVjDO2 = CaO2 − CjVO2), which help determine cerebral oxygen use, metabolic demand, and adequacy of oxygen delivery.
Monitoring of SjvO2 allows the nurse to maximize the balance between cerebral perfusion, oxygenation, and metabolism. Criteria for SjvO2 monitoring include any neurologic injury where ischemia is a threat and may include intra-operative monitoring, subarachnoid hemorrhage, and post-acute head injury with increased ICP.
Equipment
Insertion of SjvO2 monitor
Sterile towels • sterile drapes • surgical caps • gowns • sterile gloves • masks • chlorhexidine scrub • chlorhexidine solution • central venous catheter (CVC) insertion kit • 1% or 2% lidocaine without epinephrine (Xylocaine) • 5- or 10-cc syringe, with an 18G and 23G needle • 5 French percutaneous introducer • 4 French fiber-optic SjvO2 catheter • oximetric monitor with cable • 500 cc normal saline solution (heparinized or nonheparinized based on facility policy) • pressure tubing with continuous flush device • pressure bag or device • sterile occlusive dressing • sterile marker • sterile labels
Removal of SjvO2 monitor
Sterile gloves • suture removal set • sterile hemostat • sterile scissors • chlorhexidine solution • sterile occlusive dressing
Implementation
Explain the procedure to the patient and provide privacy. PCP
Wash your hands and put on sterile gloves. CDC
Using aseptic technique, prime the pressure tubing system, removing all air bubbles and maintaining sterility of the system for insertion. MFR
Position the patient with the head elevated at 30 to 45 degrees and the neck in a neutral position. Document baseline intracranial pressure (ICP).
Turn the head laterally, away from the site chosen for catheter insertion. Note and document any change in ICP.
Follow facility guidelines for the dressing procedure for insertion of central lines.
Put on new sterile gloves. Using sterile technique, open and prepare the CVC insertion kit, and add a 5 French sterile introducer and a 4 French fiber-optic SjvO2 catheter. Label all medications, medication containers, and other solutions on and off the sterile field. JCAHO
Scrub the insertion site with chlorhexidine scrub solution. CDC
Position the sterile drapes over the upper thorax and neck, exposing only the insertion site.
Assist the physician during insertion, as needed.
Monitor neurologic status, vital signs, ICP, and pain during insertion.
After the line is in place, attach the pressure tubing and confirm patency of both jugular catheter lumens by aspirating and flushing.
Obtain a lateral cervical spine or lateral skull X-tray to confirm catheter placement at the level of the jugular bulb.
Draw a jugular venous blood gas sample and perform in vivo calibration according to the manufacturer’s guidelines. MFR
Monitoring and care
Assess neurologic status, vital signs, and ICP immediately after insertion.
Record baseline parameters for continuously monitored SjvO2. Calculate AvjDO2, CeO2, and global cerebral oxygen extraction ratio (O2ER) as a baseline.
Continuously monitor SjvO2.
Verify the accuracy of the reading by drawing SjvO2 every 8 to 12 hours. The blood sample reading should be within 4% of the reading shown on the monitor.
Record SjvO2 and ICP values hourly and note trends. Assess ICP in relation to SjvO2. Notify the physician of any deviation from the trend.
Calculate CeO2 and O2ER as indicated.
Maintain a safe environment during monitoring to prevent accidental dislodgment of the catheter.
Use sedation of analgesia as indicated to maintain the monitor and enhance cerebral perfusion pressure (CPP).
Perform in vivo calibration with jugular blood gas sample as recommended by the monitor manufacturer (usually performed each shift).
Change the dressing using aseptic technique if it becomes soiled or loosened, or as indicated by facility policy for central line redressing.
Change the I.V. solution and tubing for the catheter according to facility policy. INS
Replace an SjvO2 catheter with low light intensity. Check the fiber-optic catheter for obstruction and occlusion. Aspirate the catheter until blood can be freely sampled and normal light intensity is displayed. If you can’t aspirate a blood sample, the catheter needs to be replaced.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access