IV Therapy Preparation
Selection and preparation of appropriate equipment are essential for accurate delivery of an IV solution. The type of IV administration set used depends on the rate and type of infusion desired and the type of IV solution container used. Two types of drip sets are available: the macrodrip and the microdrip. The macrodrip set can deliver a solution in large quantities at rapid rates because it delivers a larger amount with each drop. The microdrip set, used for pediatric patients and certain adult patients who require
small or closely regulated amounts of IV solution, delivers a smaller quantity with each drop.
small or closely regulated amounts of IV solution, delivers a smaller quantity with each drop.
Although plastic bags are often used to hold IV fluids, there may be times when you’ll use a bottle as a container. Glass bottles are usually used to hold medications that are unstable in a plastic bag, such as nitroglycerin. The bottle may be vented or nonvented.
Administration tubing with a secondary injection port permits separate or simultaneous infusion of two solutions; tubing with a piggyback port and a backcheck valve permits intermittent infusion of a secondary solution and, on its completion, a return to infusion of the primary solution. Vented IV tubing is selected for solutions in nonvented bottles; nonvented tubing is selected for solutions in bags or vented bottles.1 Assembly of IV equipment requires sterile technique to prevent contamination.1
Equipment
IV solution bag ▪ antiseptic pads (alcohol, tincture of iodine, or chlorhexidine-based) ▪ IV administration set ▪ IV pole ▪ medication, if necessary ▪ in-line filter, if needed.
Preparation of Equipment
Perform hand hygiene to prevent introducing contaminants during preparation.2,3,4,5,6 Verify the type, volume, and expiration date of the IV solution. Discard outdated solution. Squeeze the bag to detect leaks. If the solution is contained in a glass bottle, inspect the bottle for chips or cracks. Examine the IV solution for particles, abnormal discoloration, and cloudiness. If present, discard the solution and notify the pharmacy or dispensing department. If ordered, add medication to the solution, and place a completed medication-added label on the bag. Remove the administration set from its box, and check for cracks, holes, and missing clamps.
Implementation
Preparing the Bag
Slide the flow clamp of the administration set tubing down to the drip chamber or injection port, and close the clamp.
Place the bag on a flat, stable surface or hang it on an IV pole.
Remove the protective cap or tear the tab from the tubing insertion port (as shown below).
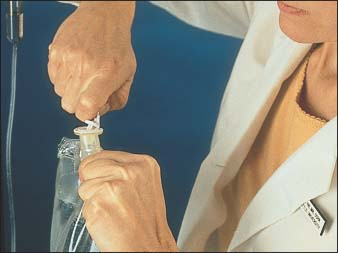
Remove the protective cap from the administration set spike.
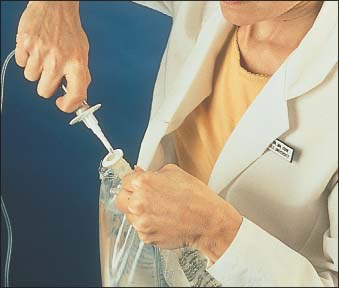
Hold the port firmly with one hand, and insert the spike with your other hand (as shown below). Be careful not to contaminate the IV bag insertion port or the IV tubing spike.
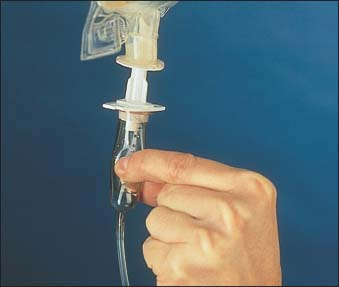
Hang the bag on the IV pole, if you haven’t already, and squeeze the drip chamber until it’s half full (as shown below).
Label the bag with the patient’s name and identification number, date and time, the bag number, ordered rate and duration of infusion, and your initials.
Preparing A Bottle
Slide the flow clamp of the administration set tubing down to the drip chamber or injection port, and close the clamp.
For a nonvented bottle, remove the bottle’s metal cap and inner disk, if present. For a vented bottle, remove the bottle’s metal cap and latex diaphragm to release the vacuum. If the vacuum isn’t intact, discard the bottle and begin again.
Place the bottle on a stable surface and disinfect the rubber stopper with an antiseptic pad.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
