Infection control
The task of preventing and controlling infectious disease became far easier in the 19th century, when Louis Pasteur and other microbiologists discovered the link between bacteria and infection. Yet, health care-associated infections (HAIs) continue to cast a shadow over patients in health care facilities.
Approximately 2 million HAIs occur annually in the United States. Their substantial morbidity and mortality cause about 80,000 patient deaths per year, raise health care costs by about $4.5 billion, and significantly prolong hospital stays. CDC For example, hospitalization for a urinary tract infection secondary to an HAI has been estimated to be about 1 to 4 days; for surgical site infections, 7 to 8 1/2 days; and for bloodstream infection, 7 to 21 days.
Studies have shown that strict adherence to infection control principles, practices, and guidelines can prevent one-third of HAIs. EB However, patients whose immune systems are compromised or suppressed might succumb to an HAI despite precautions.
In this chapter, you’ll find recommendations of best practice techniques and precautions for maintaining the highest quality infection control. Many of these recommendations are evidence-based EB. Others come from the American Hospital AssociationAHOSPA and its Patient Care PartnershipPCP, the Centers for Disease Control and PreventionCDC and its Advisory Committee on Immunization PracticesACIP, the Occupational Safety and Health AdministrationOSHA, and the Association for Professionals in Infection Control and EpidemiologyAPIC. Others are from specific manufacturers’ recommendations for the use of their products.MFR
The goal of infection control is to prevent the transmission of disease among patients, health care workers, and visitors to the health care facility. Although many factors contribute to the development of HAIs, strict adherence to your facility’s infection control policies and the practices outlined in this chapter can significantly reduce the rate of HAIs and help provide the best outcome for your patients.
Causes and incidence
Health care-associated infections (HAIs) are infections that patients acquire during the course of receiving treatment for other conditions. Health care workers can also get HAIs while performing their duties within the health care setting. HAIs result from aerobic or anaerobic bacteria, viruses, parasites, or fungi. (See Infectious diseases possibly acquired in health care facilities.) They occur most commonly in the urinary and lower respiratory tracts, bloodstream, and surgical sites. Urinary tract infections most commonly result from catheter insertion, urogenital surgery, or instrumentation. Respiratory tract infections usually result from aspiration of oropharyngeal secretions, inhalation of airborne pathogens from other patients or caregivers, contaminated ventilation equipment, or lung seeding from blood-borne pathogens. Surgical site infection may result from contamination during surgery, preexisting medical conditions, skin damage during preoperative preparation (such as shaving), or impaired blood supply.
The risk of HAIs increases with the patient’s age, underlying medical condition, length of hospitalization, and use of invasive devices.
Evolution of infection control
Infection control practices have evolved continuously since JCAHO and the American Hospital Association issued a statement in 1958 advising that every accredited
health care facility must have an infection control committee and a monitoring system as part of a formal infection control program. AHOSPA OSHA
health care facility must have an infection control committee and a monitoring system as part of a formal infection control program. AHOSPA OSHA
Infectious diseases possibly acquired in health care facilities
The Division of Healthcare Quality Promotion, a division of the Centers for Disease Control and Prevention, has identified these diseases that may be acquired by patients or health care professionals in health care facilities:
Bloodborne pathogens
Clostridium difficile
C. sordellii
Creutzfeldt-Jakob disease
Ebola (viral hemorrhagic fever)
GI infections
Hepatitis A
Hepatitis B
Hepatitis C
Human immunodeficiency virus/acquired immunodeficiency syndrome
Influenza
Methicillin-resistant Staphylococcus aureus
Norovirus
Parvovirus
Poliovirus
Pneumonia
Rubella
Severe acute respiratory syndrome
Streptococcus pneumoniae (drug resistant)
Tuberculosis
Vancomycin intermediate S. aureus
Vancomycin-resistant enterococci
Varicella (chickenpox)
Infection control timeline
This time line describes when infection control practices were implemented in hospitals and provides a brief description of various isolation categories.
1958: The Joint Commission on the Accreditation of Healthcare Organizations recommends that all accredited hospitals must have an infection control committee and implement a formal infection control program.
1970: The Centers for Disease Control and Prevention (CDC) publishes Isolation Techniques for Use in Hospitals.
1975: CDC releases an update to its 1970 isolation techniques publication.
1980: CDC releases its Guidelines for Prevention and Control of Nosocomial Infections. This guideline outlines ways to prevent or control hospital-borne pathogens.
1983: CDC releases an update to its 1975 isolation techniques publication.
1985: CDC publishes Universal Precautions.
1987: CDC establishes its recommendations for body substance isolation.
1996: CDC institutes Standard Precautions and further updates the isolation precautions.
Twelve years later, in 1970, the Centers for Disease Control and Prevention (CDC) published a guide to isolation techniques in hospitals; the guide was updated in 1975, 1983, and again in 1996. In 1980, the CDC released Guidelines for the Prevention and Control of Nosocomial Infections. The guidelines had two purposes: to disseminate advice on prevention or control of specific nosocomial infection problems and to cover the questions most frequently asked of the CDC’s Division of Healthcare Quality Promotion staff. (See Infection control timeline.)
An atmosphere of concern regarding the transmission of human immunodeficiency virus brought about the expansion of isolation guidelines to include protective measures, such as wearing gloves, masks, gowns, and goggles to guard against exposure to blood and body fluids. The expanded guidelines, released
in 1985, were called universal precautions. Body substance isolation guidelines, concerning the handling and disposal of potentially infectious body substances, were introduced in 1987.
in 1985, were called universal precautions. Body substance isolation guidelines, concerning the handling and disposal of potentially infectious body substances, were introduced in 1987.
Indications for airborne precautions
Airborne precautions are designed to reduce the risk of airborne transmission of the following infectious agents.
| Disease | Precautionary period |
|---|---|
| Chickenpox (varicella) | Until lesions are crusted and no new lesions appear |
| Herpes zoster (disseminated) | Duration of illness |
| Herpes zoster (localized in an immunocompromised patient) | Duration of illness |
| Measles (rubeola) | Duration of illness |
| Severe acute respiratory syndrome | Duration of illness; gown, gloves, and eye goggles must be worn |
| Tuberculosis (TB) — pulmonary or laryngeal, confirmed or suspected | Depends on clinical response; patient must be on effective therapy, be improving clinically (decreased cough and fever and improved findings on chest radiograph), and have three consecutive negative sputum smears collected on different days, or TB must be ruled out |
In 1996, the CDC instituted standard precautions, which combined major features of blood and body fluid precautions with practices regarding body substance isolation. Standard precautions recommend that health care workers wear appropriate protective clothing or use appropriate protective equipment when the duty being performed requires or may involve contact with any body substance, mucous membrane, or broken skin. The CDC also added three other categories called transmission-based precautions — airborne precautions, droplet precautions, and contact precautions.
Supportive references
Centers for Disease Control and Prevention. Isolation Techniques for Use in Hospitals, 2nd ed. Washington, D.C.: U.S. Department of Health and Human Services, 1975. HHS publication no. (CDC) 76-8314.
Garner, J.S. “Hospital Infection Control Practices Advisory Committee Guideline for Isolation Precautions in Hospitals,” Infection Control and Hospital Epidemiology 17:53-80, January 1996. www.cdc.gov/ncidod/hip/isolat/isopart2.htm. EB
Joint Commission on Accreditation of Healthcare Organizations. Comprehensive Accreditation Manual for Hospitals. Oakbrook Terrace, Ill.: JCAHO, 2005. www.jcaho.gov.
Airborne precautions CDCEB1
Airborne precautions, used in addition to standard precautions, prevent the spread of infectious diseases transmitted by droplet nuclei 5 μm or smaller that are breathed, sneezed, or coughed into the environment. (See Indications for airborne precautions.) Diseases in this category include the former categories of acid-fast bacillus isolation and respiratory isolation.
To effectively guard against the spread of infection from an airborne transmitted infection, an isolation patient requires a monitored negative-pressure room with the door kept closed to maintain the proper air pressure balance in the isolation room, the anteroom, and the adjoining hallway or corridor. The air should undergo 12 air exchanges per hour for new construction and 6 air exchanges per hour for construction before
2001. EB2 It should be appropriately discharged directly to the outside of the building or filtered through high-efficiency particulate air (HEPA) filtration before it’s circulated to other areas of the health care facility. If for some reason a private room isn’t available, patients infected with the same disease can share a room, but consultation with infection control professionals is advised before patient placement.
2001. EB2 It should be appropriately discharged directly to the outside of the building or filtered through high-efficiency particulate air (HEPA) filtration before it’s circulated to other areas of the health care facility. If for some reason a private room isn’t available, patients infected with the same disease can share a room, but consultation with infection control professionals is advised before patient placement.
All persons who enter the room must wear respiratory protection. In 1993, the Centers for Disease Control and Prevention (CDC) published tuberculosis guidelines recommending the use of a disposable respirator, such as an N95 respirator or HEPA respirator; a reusable respirator, such as a HEPA respirator; or a powered air-purifying respirator (PAPR) that provides protection against microorganisms transmitted by airborne droplet nuclei. Regardless of the type of respirator used, the health care worker must make sure that the respirator properly fits her face, covering the mouth and nose, each time she wears it. Fit testing of all respirators ensures proper fit. OSHA If the patient must leave the room for an essential procedure, he should wear a surgical mask to cover his nose and mouth while out of the room, and personnel in the area where the patient is going should be notified and instructed to take airborne precautions.
Equipment
Respirators (either disposable N95 or HEPA respirators or reusable HEPA respirators or PAPRs) • surgical masks • isolation door card • other personal protective equipment as needed for standard precautions
Gather additional supplies needed for routine patient care, such as a thermometer, stethoscope, and blood pressure cuff.
Preparation of equipment
Keep airborne precaution supplies outside the patient’s room in a cart or anteroom.
Implementation
Situate the patient in a negative-pressure room with the door closed. If possible, the room should have an anteroom, and it should be possible to monitor the negative pressure. If necessary, two patients with the same infection may share a room. Explain isolation precautions to the patient and his family.
Keep the patient’s door (and the anteroom door) closed at all times to maintain the negative pressure and contain the airborne pathogens. Put an airborne precautions sign on the door to notify anyone entering the room.
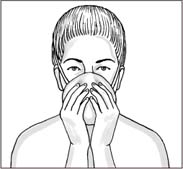
Checking the respirator seal
Before using a respirator, always check the respirator seal. To do this, place your hands over the respirator and exhale. If you feel air leaking around your nose, adjust the nosepiece. If air leaks at the respirator’s edges, adjust the straps along the side of your head. Recheck respirator fit after this adjustment.
 |
Put your respirator on according to the manufacturer’s directions. MFR Adjust the straps for a firm but comfortable fit. Check the fit. (See Checking the respirator seal.)
Instruct the patient to cover his nose and mouth with a facial tissue while coughing or sneezing.
Tape an impervious bag to the patient’s bedside so the patient can dispose of facial tissues correctly.
Make sure that visitors wear respiratory protection while in the patient’s room.
Limit the patient’s movement from the room. If he must leave the room for essential procedures, make sure he wears a surgical mask over his nose and mouth. Notify the receiving department or area of the patient’s isolation precautions so that the precautions will be maintained and the patient can be returned to his room promptly.
All negative-pressure rooms require constant monitoring usually via electronic devices. CDC When the monitor’s alarm sounds, it indicates a problem with negative pressure.MFR
Special considerations
Before leaving the room, remove gloves (if worn), and wash your hands. Remove your respirator outside the patient’s room after closing the door.
Depending on the type of respirator and recommendations from the manufacturer, follow your facility’s policy and either discard your respirator or store it until the next use. If your respirator is to be stored until the next use, store it in a dry, well-ventilated place (not a plastic bag) to prevent microbial growth. Nondisposable respirators must be cleaned according to the manufacturer’s recommendations. MFR
Nursing diagnoses
Risk for injury
Risk for loneliness
Expected outcomes
The patient will:
help identify and apply safety measures to prevent injury
develop strategies to maintain safety
identify feelings of loneliness
identify ways to socialize within the confines of isolation.
Documentation
Record the need for airborne precautions on the nursing care plan and as otherwise indicated by your facility. Document initiation and maintenance of the precautions, the patient’s tolerance of the procedure, and any patient or family teaching. Also document the date airborne precautions were discontinued.
Supportive references
Garner, J.S. “Hospital Infection Control Practices Advisory Committee Guideline for Isolation Precautions in Hospitals,” Infection Control and Hospital Epidemiology 17(1):53-80, January 1996. www.cdc.gov/ncidod/hip/isolat/isopart2.htm. EB1
“Guidelines for Environmental Infection Control in Health Care Facilities,” MMWR Morbidity and Mortality Weekly Report 52(RR-10):1-42, June 2003.EB2
Contact precautions CDC
Contact precautions prevent the spread of infectious diseases transmitted by direct or indirect contact with the patient (skin-to-skin), patient-care items (bedpans, urinals), or indirect contact with surfaces in the patient’s room that are contaminated with the infectious microorganism. (See Indications for contact precautions.)
The Centers for Disease Control and Prevention (CDC) recommends that contact precautions apply to patients who are known or suspected to be infected or colonized (presence of microorganism without obvious clinical signs and symptoms of infection) with epidemiologically important organisms that can be transmitted by direct or indirect contact. Effective contact precautions require a private room. If no private room is available, two patients infected with the same (but no other) microorganism can share a room.
CDC guidelines advise considering the epidemiology of the microorganism and the patient population and consulting infection control professionals before patient placement.
Anyone having contact with the patient, the patient’s support equipment, or items soiled with the patient’s bodily fluids should wear clean, nonsterile gowns and gloves. Gloves should be changed after contact with infective material that may contain high concentrations of the microorganism, such as fecal material or wound drainage. A gown and gloves should be removed before leaving the patient’s room. Thorough hand washing with an antimicrobial agent or waterless antiseptic agent and proper handling and disposal of contaminated items are also essential in maintaining contact precautions.
Patient transport should be limited to essential purposes only. If the patient is transported, make sure that precautions are maintained to decrease the risk of transmission to other patients and contamination of environmental surfaces.
When possible, medical and noncritical patient-care equipment (I.V. pumps, monitors) should be used for only one patient. If sharing equipment is unavoidable, then items must be properly cleaned or disinfected before use with another patient.
Indications for contact precautions
Contact precautions are designed to reduce the risk of transmitting infectious agents, by direct or indirect contact, such as the ones listed here.
| Disease | Precautionary period |
|---|---|
| Acute viral (acute hemorrhagic) conjunctivitis | Duration of illness |
| Clostridium difficile enteric infection | Duration of illness |
| Diphtheria (cutaneous) | Duration of illness |
| Enteroviral infection, in diapered or incontinent patient | Duration of illness |
| Escherichia coli disease, in diapered or incontinent patient | Duration of illness |
| Hepatitis A, in diapered or incontinent patient | Duration of illness |
| Herpes simplex virus infection (neonatal or mucocutaneous) | Duration of illness |
| Impetigo | Until 24 hours after initiation of effective therapy |
| Infection or colonization with multidrug-resistant bacteria | Until off antibiotics and culture is negative |
| Major abscesses, cellulitis, or decubiti | Until 24 hours after initiation of effective therapy |
| Parainfluenza virus infection, in diapered or incontinent patient | Duration of illness |
| Pediculosis (lice) | Until 24 hours after initiation of effective therapy |
| Respiratory syncytial virus infection, in infants and young children | Duration of illness |
| Rotavirus infection, in diapered or incontinent patient | Duration of illness |
| Rubella, congenital syndrome | Precautions during any admission until infant is age 1, unless nasopharyngeal and urine cultures negative for virus after age 3 months |
| Scabies | Until 24 hours after initiation of effective therapy |
| Shigellosis, in diapered or incontinent patient | Duration of illness |
| Smallpox | Duration of Illness; requires airborne and contact precautions |
| Staphylococcal furunculosis in infants and young children | Duration of illness |
| Viral hemorrhagic infections (Ebola, Lassa, Marburg) | Duration of illness |
| Zoster (chickenpox, disseminated zoster, or localized zoster in immunodeficient patient) | Until all lesions are crusted; requires airborne precautions |
Equipment
Gloves • gowns or aprons • masks, if necessary • isolation door card • plastic bags
Gather additional supplies, such as a thermometer, stethoscope, and blood pressure cuff.
Preparation of equipment
Keep contact precaution supplies outside the patient’s room in a cart or anteroom.
Implementation CDC
Situate the patient in a single room with private toilet facilities and an anteroom, if possible. If necessary, two patients with the same (but no other) infection may share a room. Explain isolation procedures to the patient and his family.
Instruct visitors to wear gloves and a gown while visiting the patient and to wash their hands after removing the gown and gloves.
Place a contact precautions card on the door to notify anyone entering the room.
Wash your hands before entering and after leaving the patient’s room and after removing gloves.
Place laboratory specimens in impervious, labeled containers, and send them to the laboratory at once. Attach requisition slips to the outside of the container.
Place items that have come in contact with the patient in a single impervious bag, and arrange for disposal or disinfection and sterilization. OSHA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


