 Evidence Base
Evidence Base
At the beginning of this epidemic, AIDS affected men who had sex with men (MSM), persons who shared needles when using intravenous drugs, persons who received large-volume transfusions of blood or blood products, heterosexual partners of infected persons, and children of infected mothers. Today, the majority of new HIV infections are found among MSM, and disproportionately among racial minorities and transgender persons. Others at risk include injection drug users (IDU), African Americans, those having heterosexual sex, and women. Mother-to-child transmission of HIV in the United States is reduced to <2%, owing to aggressive HIV testing, antiretroviral treatment of pregnant women, and other interventions in the intrapartum and postpartum period. Transmission from donor blood is very rare.
HIV enters the body through unprotected sexual contact or blood-to-blood transmission, then targets immune cells that carry CD4+ receptors, namely T cells and macrophages. Once inside the host cell, HIV uses the cell’s components as a factory to reproduce. Eventually, reproduction of HIV within the host cell ruptures the cell membrane, releasing into the plasma more HIV virions that bind to and disable other CD4+ cells.
HIV infection may be viewed as a chronic condition if patients strictly adhere to treatment and care. However, some patients have difficulty managing highly structured medication and appointment regimens and need encouragement or
special interventions to support their treatment plan. Nursing care is important to tailoring a plan with the patient that optimizes her or his chances for successful and enduring treatment.
 NURSING ALERT
NURSING ALERT
Approximately 50% to 90% of patients who become infected experience some symptom of primary HIV infection around 2 to 4 weeks after exposure to HIV. Typical symptoms include fever, adenopathy, pharyngitis, and rash. Many new HIV infections evade diagnosis because symptoms of acute HIV infection mimic other common infections. Recently, more persons who present to urgent care with these symptoms receive HIV testing and diagnosis. During this phase, the immune system is compromised by a sudden decrease in T4 helper cells and an increase in HIV viral load for a brief period before settling into a new baseline.
Seroconversion occurs when the person has formed enough antibodies to HIV that the serologic test is positive. This usually occurs 4 to 6 weeks after acute HIV infection.
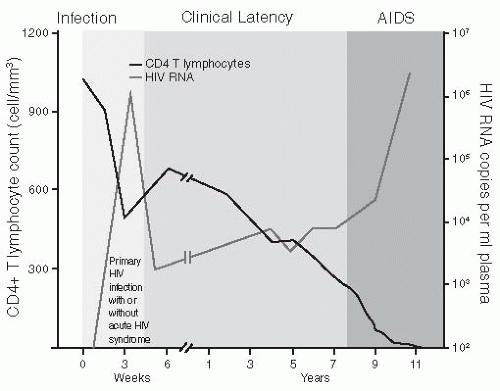
The CDC provides a mechanism for staging HIV infection and defining the continuum to AIDS using clinical findings and the CD4+ count (see Table 29-1). A normal CD4+ count is 800 to 1,000/mm3. Because HIV replication destroys CD4+ cells, the number of these cells diminishes slowly over time.
Waning immunity in persons with untreated and treated HIV infection allows development of frequent infections, severe opportunistic infections, and malignancies. These usually appear years after exposure to HIV (see Figure 29-1).
Table 29-1 AIDS Surveillance Case Definition for Adolescents and Adults | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
 NURSING ALERT
NURSING ALERT DRUG ALERT
DRUG ALERT
Pulmonary manifestations.
Persistent or acute cough, with or without sputum production, shortness of breath, chest pain, fever.
From Pneumocystis jiroveci pneumonia (PCP), bacterial pneumonia (community-acquired pneumonia), Mycobacterium tuberculosis, disseminated Mycobacterium avium complex, Aspergillus, Pseudomonas, cytomegalovirus (CMV), Histoplasma, Kaposi’s sarcoma, lung cancer, lymphoma, Cryptococcus, Legionella, or other pathogens and malignancies.
GI manifestations.
Diarrhea, weight loss, anorexia, abdominal cramping, feeling of fullness, rectal urgency (tenesmus).
From enteric pathogens or malignancies including Salmonella, Shigella, Campylobacter, Entamoeba histolytica, C. difficile, CMV, M. avium complex, herpes simplex, Strongyloides, Giardia, Cryptosporidium, Isospora belli, Chlamydia, lymphoma, Kaposi’s sarcoma, and others.
Feeling of difficulty swallowing or food feeling stuck substernally upon swallowing, usually caused by Candida esophagitis. Also consider an esophageal ulcer caused by herpes simplex, CMV, or aphthous stomatitis.
Oral manifestations.
Appearance of oral lesions, white plaques on oral mucosa, particularly in the posterior pharynx, and angular cheilitis from Candida albicans of mouth.
Vesicles with ulceration from herpes simplex virus.
White, thickened lesions on lateral margins of tongue from oral hairy leukoplakia.
Oral warts due to human papillomavirus.
Periodontitis progressing to gingival necrosis.
Painful, solitary lesions with raised margins—aphthous ulcers of unclear etiology.
Appearance of flat or nodular purple lesions on the hard or soft palate, buccal mucosa, posterior pharynx from Kaposi’s sarcoma.
CNS manifestations.
Cognitive, motor, and behavioral symptoms may be caused by HIV encephalopathy, AIDS dementia, acute infection, toxicity from alcohol or drugs, adverse reaction to medication, psychiatric condition, and other causes.
Acute symptoms of infection with fever, malaise, headache, and/or mental status change, seizure, hemiparesis, abnormal gait or speech may be caused by toxoplasmosis, cryptococcal meningitis, herpes virus infections, CMV encephalitis, progressive multifocal leukoencephalopathy, CNS lymphoma, neuro syphilis, or other pathogens and malignancies.
May also have sensory symptoms (distal symmetrical polyneuropathy)—demonstrated by numbness, tingling, and neuropathic pain of the feet or hands.
Ocular manifestations.
Seeing floaters in the visual field, flashes of light or sudden loss of a visual field in persons with <100 CD4/mm3 due to CMV retinitis, a sight-threatening infection that requires urgent evaluation by an ophthalmologist.
Blurry vision, dry eyes, double vision, or swelling of the eyelid or conjunctiva, caused by bacterial or viral conjunctival infection, adverse reaction to medication, syphilis, Kaposi’s sarcoma, or other pathogens and malignancies.
Malignancies.
Kaposi’s sarcoma (AIDS-defining cancer) (ADC).
Non-Hodgkin’s lymphoma (ADC) and other lymphomas (non-ADC).
Cervical cancer (ADC).
Liver cancer (non-ADC).
Lung cancer (non-ADC).
Anal cancer (non-ADC).
Skin manifestations.
Pruritic rash with raised, erythematous papules, located on surfaces that contain hair follicles known as HIV folliculitis.
Abscesses caused by methcillin-sensitive and methcillinresistant S. aureus infections or other common skin pathogens.
Pruritic nodular rash located on any skin surface known as prurigo nodularis.
Purple, flat, or nodular lesions located on any skin surface including soles of feet and palms of hands—likely to be Kaposi’s sarcoma and should be biopsied.
 Evidence Base
Evidence Base Evidence Base
Evidence Base
Enzyme-linked immunosorbent assay (ELISA)—serologic test for detecting antibody to HIV. Western blot test is used to confirm a positive result on ELISA.
Rapid test (serology or oral sample)—15- to 40-minute result; used in settings where patient is less likely to return for result such as mobile van, emergency department, urgent care center, or STD clinic.
Antibodies to HIV generally take 2 to 12 weeks to develop. Therefore, a negative-HIV antibody test may catch a person within this 12-week time frame to HIV seroconversion, known as the window period.
Occasionally, an ELISA screen may yield an indeterminate result by Western blot.
The cause of an indeterminate result may be early HIV seroconversion, HIV vaccine, infection with O strain or HIV-2, or a false-positive in a low-risk individual.
The test should be repeated at 1, 2, and 6 months until Western blot becomes positive or there is no longer suspicion of HIV infection. If a conclusive test is needed quickly, HIV RNA viral load testing can be done.
The CDC recommends opt-out testing for HIV for persons age 13-64, which requires general medical consent for testing and optional prevention counseling.
Lymphocyte panel may show decreased CD4+ count. In early infection or in long-term nonprogressors, CD4+ count may be normal.
A complete blood count (CBC) may show anemia, low white blood cell count, and/or decreased platelets.
Presence of indicator infection through microbiology or serology testing (eg, PCP, candidiasis of esophagus, herpes zoster).
HIV viral load testing is a measure of the amount of HIV in the blood. High viral loads (>750,000) tend to be found in acute seroconversion and late infection, but also occur in patients who have a concurrent infectious process. A viral load test result can be undetectable, meaning the amount of virus is less than the limit of the test, which may be either <400 or <50 copies/mL.
Resistance testing is done to determine if the patient is infected with a drug-resistant virus. The test uses genotypic assays that amplify the HIV virus and look for mutations in the viral genotype, which represent changes that block or impair the effectiveness of specific antiretroviral agents.
 NURSING ALERT
NURSING ALERT Evidence Base
Evidence Base
Antiretroviral treatment (ART) has a four-part purpose: (1) to decrease HIV viral burden, increase immune function, (2) to protect against morbidity, (3) to increase quality of life and chance for survival, and (4) to prevent HIV transmission. Successful treatment requires strict adherence to medication, which means taking at least 90% of doses. Before initiating antiretrovirals, patients should be assessed for readiness to start as treatment is lifelong. However, some trepidation is expected and is not a complete bar to initiating treatment. See Table 29-2.
Some opportunistic infections can be prevented by prophylactic medication. All persons with CD4 <200 cells/mm3 must receive prophylaxis against PCP. Those who have CD4 < 100 cells/mm3 and are Toxoplasma gondii serum IgG+ must receive prophylaxis against toxoplasmosis. And those who have CD4 < 50 cells/mm3 must receive prophylaxis against Mycobacterium avium complex, after initial culture for M. avium intracellulare is negative.
Treatment is available for most opportunistic infections and other infections associated with AIDS. After initial treatment of an opportunistic infection, the patient may need to remain on continued suppressive therapy to prevent recurrence. This is known as secondary prophylaxis. Prophylaxis may be discontinued after a period of sustained immune reconstitution beyond the threshold of vulnerability for the particular opportunistic infection. See Table 29-3.
Care and treatment of HIV infection requires the expertise of many specialties including infectious infection, renal, pulmonary, gastroenterology, neurology, obstetrics and gynecology, dentistry, dermatology, cardiology, surgery, psychiatry, nursing, nutrition, and social work.
Table 29-2 Antiretroviral Therapy* | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree