Cutaneous Malignancies
Grace Chung
OBJECTIVES
After studying this chapter, the reader will be able to:
Distinguish between premalignant and malignant skin lesions by different growth patterns and clinical presentations.
Identify the predisposing factors of cutaneous malignancies.
Describe the relationship of ultraviolet radiation exposure and genetic mutations in development of cutaneous malignancies.
Discuss the various treatment options available for patients diagnosed with cutaneous malignant diseases based on clinical and histologic assessment.
KEY POINTS
Tumors and pigmented lesions of the skin may be benign, premalignant, or malignant.
Regularly performed visual skin examinations and photoprotective behaviors are the most indispensable and cost-effective preventive measures against development of skin cancers.
Persistent hyperkeratotic and indurated actinic keratosis lesions should be considered for skin biopsy to rule out skin cancers.
Squamous cell carcinoma is locally invasive and has potential to metastasize.
Pearly appearance with telangiectasias is the most common presentation of basal cell carcinoma. A pigmented basal cell carcinoma, however, may resemble other types of growth lesions (e.g., nodular melanoma, pigmented seborrheic keratosis, melanocytic nevus).
The four types of malignant melanoma include superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, and acral lentiginous melanoma. The prognosis of a malignant melanoma depends on tumor thickness, depth of invasion, and distant metastasis.
Cutaneous T-cell lymphoma is a rare type of cancer that originates in white blood cells and typically appears in severely itch skin lesions. Patients with chronic skin rash that delays improvement, progresses through different stages of lesions, and repeated cycles of regression and recurrence should be evaluated for cutaneous T-cell lymphoma.
Sézary syndrome is an aggressive form of leukemic cutaneous T-cell lymphoma variant.
Kaposi sarcoma is a multicentric, lymphatic, and vascular epithelial hyperplasia and not a sarcoma of mesenchymal origin.
ACTINIC KERATOSIS
I. OVERVIEW
Actinic keratosis (AK), also called solar keratosis or senile keratosis (nonrecommended synonym), is a premalignant neoplasm of the epidermis. AK presents as a solitary or multiple lesions on sun-exposed areas, and greater than 80% occur on the head, neck, forearms, and dorsal hands. History of AK correlates with increased risk for developing invasive squamous cell carcinomas (SCCs).
A. Definition: AK is a premalignant, atypical proliferation of mutated keratinocytes arising from the basal layer and remaining in the epidermis.
B. Incidence:
1. One of the most commonly seen problems in dermatology visits, affecting more than 58 million people in the United States.
2. The incidence varies by skin type, geographic location, and amount of ultraviolet (UV) radiation exposure.
C. Etiology:
1. Chronic occupational and recreational UV radiation exposure
2. Genetic predisposition: fair skin and family history of skin cancers
3. Genetic disorders: ineffective or interference of DNA repair against skin sun damage (i.e., xeroderma pigmentosum, Bloom syndrome, and Rothmund-Thomson syndrome)
4. Gender (male > female), advancing age, and geographic location
5. History of serious sunburn
6. Immunosuppression in organ transplant recipients and immunomodulatory medication (biologic) users in autoimmune disorders
D. Pathogenesis:
1. Chronic UV radiation exposure, mostly UVB (290 to 320 nm), induces mutation of epidermal keratinocyte DNA.
2. Keratinocyte DNA mutation in the p53 tumor suppressor gene located on chromosome 17p132 involves in cell cycle regulation, apoptosis (programmed cell death process), and DNA repair.
3. Mutated p53 gene leads to prolonged cell life span and delayed cell apoptosis, resulting in proliferation and clonal expansion of atypical keratinocytes and formation of AKs.
II. CLINICAL VARIANTS
A. Common AK: few millimeters to 2 cm in diameter, pink or erythematous, keratotic papule or plaque (Figure 16-1)
B. Hypertrophic AK (HAK): tan, white, or gray, thick or exophytic (outward-growing) scaly papule or plaque, with erythematous base (Figure 16-2)
C. Atrophic AK: smooth pink or erythematous macule or patch, with absent keratotic scale
D. Pigmented AK: keratotic scale lying over hyperpigmented macule or patch
E. Cutaneous horn: keratotic projection (exophytic), with the mound of compact keratin resembling the shape of a cone or spicule
F. Actinic cheilitis (solar cheilosis): rough keratotic papule or plaque occurring on the lip; may be cracking (fissuring) or ulcerating
III. LABORATORY AND DIAGNOSTIC TESTS
A. Often clinically diagnosed by visual inspection and palpation.
B. Dermoscopy using handheld device to magnify and illuminate lesions for making clinical diagnosis.
C. Skin biopsy is indicated when diagnosis is uncertain, to get definitive diagnosis and rule out malignancy or differentiate from other nonmalignant growth lesions.
IV. DIFFERENTIAL DIAGNOSIS
Use a fingertip as the diagnostic tool to distinguish AK lesions from common flaky scales on dry skin surface.
A. Inflamed seborrheic keratosis
B. Pityriasis rubra pilaris
 FIGURE 16-1. Actinic keratosis: pink keratotic plaques on this 58-year-old man’s forearm. (Courtesy of Grace Chung.) |
C. Porokeratosis
D. Prurigo nodularis
E. Psoriasis
F. Seborrheic dermatitis
G. Squamous cell carcinoma
H. Superficial basal cell carcinoma (BCC)
I. Viral wart
V. TREATMENT MODALITIES
A. Cryotherapy: most common, first-line treatment option with a cure rate of 98.8%
B. Electrodessication and curettage: ideal treatment for hyperkeratotic AKs and those resembling the presentation of invasive SCC
C. Topical 5-fluorouracil (5-FU): interferes with DNA and RNA synthesis of the mutated keratinocytes by blocking the methylation reaction of deoxyuridylic acid to thymidylic acid
D. Imiquimod (immune response modifier): induces synthesis and release of cytokines such as interferon-tumor necrosis factors (TNF) and interleukins
E. Prevention: sun avoidance, use of sunscreen, photoprotective clothing, and self-screening exam
VI. PROGNOSIS
AKs are premalignant, but they have potential to progress into malignant lesions. Approximately 65% of all SCC and 36% of all BCC are transformed from preexisting AKs.
Actinic Keratosis
Rough and scaly actinic keratosis (AK) lesions are easier felt than seen.
AK is a precancerous lesion. Patient should not get confused with the number of AK lesions removed by various types of treatment methods in reporting the history of skin cancer.
Reasons to remove AK lesions on the skin include cosmetic concerns (unsightliness), frequent irritation, and potential risk of developing into an SCC (rarely BCC).
Photoprotection is the key in preventing sun damage and future occurrence of AK.
BASAL CELL CARCINOMA
I. OVERVIEW
BCC is the most common nonmelanoma skin cancer (NMSC) in the United States. UV radiation exposure is considered the biggest contributing risk factor for BCC as evidenced by the common sites of occurrence on sun-exposed areas such as the scalp, face, neck, chest, and distal parts of extremities. The malignant nature of BCC is demonstrated by expansive growth, destruction, and invasion of local structures including bone. BCC often remains local with low metastatic potential, and it’s highly curable.
A. Definition: BCC is a malignant epithelial neoplasm arising from the basal cells (in the basal layer) of the epidermis and its appendages.
B. Incidence:
1. Over 3.5 million cases of NMSC among more than 2 million people annually.
2. Highest incidence among Caucasians and individuals with fair skin, approximately 75% to 80% of NMSC.
3. Greater than 70% occurrences are on the face, 15% on the trunk, and rare occurrences on non-sun-exposed areas including genital or perianal areas.
C. Etiology:
1. UV radiation exposure
a. Intermittent episodes of intense UV exposure and recreational sun exposure at any age.
b. Depletion of earth’s protective ozone layer allows dangerous UV rays to penetrate the atmosphere.
2. Ionizing radiation exposure through occupation or therapeutic radiation for disorders such as facial acne, tinea capitis, psoriasis, eczema, or skin cancer.
3. Arsenic and carcinogenic exposures from contaminated drinking water, seafood, agricultural insecticides, or pharmacological mixtures (Fowler solution).
4. Genetic disorders and DNA repair capability:
a. Nevoid basal cell carcinoma syndrome (NBCCS) (also known as Gorlin syndrome)—a rare autosomal dominant genetic disorder, caused by the germline mutations (Figure 16-3)
 FIGURE 16-3. Nevoid basal cell carcinoma syndrome. (From DeVita, V. T., et al. (2008). DeVita, Hellman, and Rosenberg’s cancer: Principles and practice of oncology. Philadelphia, PA: Wolters Kluwer.) |
(1) Affected individuals have multiple developmental anomalies in body systems (e.g., skin, bone, endocrine, and nervous system), unusual facial appearance, and predisposition for tumors including multiple BCCs at an early age.
b. Xeroderma pigmentosum (XP)—an autosomal recessive genetic disorder with defective DNA repair mechanism. Inability to repair UV radiation-induced DNA damage and mutations results in development of BCC.
5. BCC may arise in nevus sebaceous, a congenital hamartoma of the skin.
D. Pathogenesis:
1. The mutations in PTCH1, PTCH2, SMO and SUFU genes are well known to associate with the development of BCC. Over 70% of BCC development is caused by alteration in PTCH1 gene on chromosome 9q. PTCH1 is a tumor suppressor gene, functioning as the controller of proliferation and differentiation.
2. PTCH1 gene mutation causes loss of normal function and disruption in signaling pathways, resulting in uncontrolled growth in BCC tumorigenesis.
3. The mutations in P53 gene (early during carcinogenesis) and the MC1R gene are uncommonly involved in the development of BCC.
4. UV irradiation-induced skin inflammation: UV exposure results in inflammation of the skin, which leads to increased synthesis of prostaglandin and induction of cyclooxygenase-2 (COX-2). The key role of COX-2 inhibitors induced by the inflammation is to decrease the incidence of carcinogenesis in NMSC.
5. Langerhans cells, found in the basal layer of the epidermis, are functionally impaired by UVB and PUVA treatment leading to morphologic changes within the cell.
a. PUVA, PUVB, broadband UVB, and narrow band UVB phototherapy are types of UV radiation treatments for many severe skin diseases.
(1) PUVA is a UV radiation treatment using a photosensitizing agent called Psoralens (P), such as methoxsalen (8-methoxypsoralen), 5-methoxypsoralen, and trisoralen orally or topically. Once the medication is applied (or taken orally 2 hours prior to the treatment), expose the treatment areas of the skin to UVA (long UV wavelength, 320 to 400nm) or UVB (290 to 320nm) in a therapeutic light box, a cabinet containing multiple fluorescent light bulbs to administer controlled amount of UV radiation, 2 to 3 times a week until optimal therapeutic outcomes are achieved.
(2) Since 2008 however, lack of supplies in Psoralens caused infrequent use of PUVA or PUVB; currently the most common form of phototherapy is Narrowband UVB phototherapy (NB-UVB). NB-UVB (311 to 312mn) is known to have lower risk associated with phototherapy in comparison to formerly used broadband UVB (290 to 320nm).
(3) Patients must use protective eyewear and cover sensitive skin areas (i.e. face and genitalia) for whole body exposure to limit UV radiation associated irritations, burning, and other damages during the treatment. Currently there are localized treatment instruments available for scalp, extremities, hands and feet.
II. CLINICAL VARIANTS
A. Superficial BCC (Figure 16-4): pearly pink, reddish, or eczematous, with a slightly scaling patch or slightly raised plaque.
 FIGURE 16-5. Nodular basal cell carcinoma. (From Lugo-Somolinos, A., et al. (2011). VisualDx: Essential dermatology in pigmented skin. Philadelphia, PA: Wolters Kluwer.) |
B. Papulonodular BCC (Figure 16-5): pearly, shiny, or semitranslucent pink papule or nodule with a crater-like central umbilication and visible surrounding telangiectasia.
C. Sclerotic or morpheaform BCC (Figure 16-6): pale, atrophic, occasionally eroded, or crusting, cicatricial-appearing plaque.
D. Pigmented BCC (Figure 16-7): shiny papule, nodule, or plaque with focally speckled or entirely dark brown, blueblack, or black pigmentation while having pearly, semitranslucent rolled border at the base.
E. Basosquamous cell carcinoma: rarely occurring tumor but aggressive in nature and often classified as an SCC. This type must be confirmed by skin biopsy and histologic study.
 FIGURE 16-6. Morpheaform basal cell carcinoma. (From Khan, F. M., & Gerbi, B. J. (2011). Treatment planning in radiation oncology. Philadelphia, PA: Wolters Kluwer.) |
III. LABORATORY AND DIAGNOSTIC TESTS
A. Clinical evaluation and skin biopsy for a confirmation of diagnosis.
B. Skin biopsy is highly recommended for patients with atypical features of a lesion for BCC, no prior history of BCC, or other risk factors contributing to possible recurrence of BCC.
IV. DIFFERENTIAL DIAGNOSIS
A. Amelanotic melanoma
B. Dermal nevi
C. Eczema
D. Keratoacanthoma
E. Nodular melanoma
F. Nummular eczema
G. Psoriasis
H. Scar/cicatricial plaque
I. Squamous cell carcinoma
V. TREATMENT MODALITIES
Treatment modalities are decided by the location and size of the tumor.
A. Cryosurgery
1. A noninvasive procedure using liquid nitrogen.
2. Useful in small superficial BCC treatment on trunk and extremities.
3. Liquid nitrogen is administered by cone spray apparatus or by use of cryoprobes that conduct cold evenly.
4. May result in postinflammatory hypo- or hyperpigmentation (PIH).
5. No specimen available for evaluation of margins.
B. Electrosurgery (electrodessication and curettage)
1. Local anesthesia, minimal equipment, and quick office procedure.
2. Useful in treatment of well-differentiated primary BCCs located on trunk and extremities and on patients who are not candidates for invasive surgical procedures.
3. Disadvantages include lack of specimen for margin evaluation and possible hypertrophic scar development.
4. Contraindicated in patients with pacemaker or defibrillator implant, BCCs 2 cm or larger, recurrent BCC, or a BCC located in high-risk location for recurrence.
5. Electrodessication and curettage offers cure rate of 97% to 98%.
C. Carbon dioxide laser (CO2)
1. May be used in conjunction with a curette in place of electrocautery.
2. Advantages include minimal nonspecific thermal injury to adjacent cells with more rapid healing and less pain postoperatively.
D. Surgical excision
1. Office procedure using local anesthetic
2. Useful for well-differentiated primary BCC lesions
3. Provides specimen for random evaluation of surgical margins
4. Time-consuming procedure requiring surgical skill, assistants, and more equipment
5. Highly efficacious with cure rate of 96.8% to 99% depends on the types, sizes, depths, and locations of the lesions.
E. Radiation therapy
1. Superficial x-ray or high-energy electron beam radiation.
2. Useful for BCCs on the head and neck, with chance of metastasis
3. May cause radionecrosis over bone
F. Mohs micrographic surgery, named after Frederic Mohs, MD, is defined as excision of skin cancer with histological margin control of deep and peripheral margins.
1. Office procedure using local anesthesia.
2. Indications:
a. Primary BCC on cosmetic sensitive areas (e.g., face and ears).
b. Morpheaform BCC or tumors with indistinct margins.
c. Incompletely excised and recurrent BCC.
d. Certain anatomic areas best treated by the precision of Mohs surgery include central “H” zone of the face comprising the upper lip, nose, medial canthus, and temple.
3. Tumor is debulked and a layer of tissue is removed to be processed for margin evaluation in an on-site laboratory.
4. Tissue specimen is marked with color-coding inks to maintain the orientation of the specimen and the defect (surgical site).
5. The specimen is then frozen, sliced, and fixed on glass slides and treated in series of chemicals for staining.
6. Surgeon or dermatopathologist reads slides observing the base and epidermal edges of the specimen for the total clearance of the tumor; if BCC persists at one or more color-coded margins, another layer from the corresponding site(s) is harvested from the defect. (Same steps are repeated until the margins are free of tumor.)
7. Precise margin control and conservative removal of the benign skin surrounding the tumor offer advantage in cosmetic sensitive areas and higher cure rate.
8. Disadvantages include possible lengthy procedures with waiting periods; requires special training of surgeon, assistants, and technicians and specialized equipment.
G. Other treatment modalities
1. Topical 5-fluorouracil and imiquimod are effective treatments for superficial BCC and multifocal BCC. May also be used as pretreatment method prior to Mohs or excision surgery for ill-defined BCC lesions on diffuse sun-damaged skin.
2. Photodynamic therapy (PDT) for superficial BCC, which selectively destroys tumor cells by the administration of a photosensitizing topical agent that is activated by light creating oxygen products capable of cell destruction.
a. Beneficial for patients with nevoid BCC syndrome
VI. PROGNOSIS
BCCs’ viability is dependent on the integumentary system (e.g., pilosebaceous units and loose connective tissue stroma). Therefore, they have low chance of metastasis and highly curable. Occasionally occurring metastases are associated with long-standing history of neglecting and untreating a large, ulcerating, and aggressively growing, nonhealing lesions.
Basal Cell Carcinoma
UV radiation exposure, especially a person’s life style, habits, and susceptibility to solar radiation, is the biggest contributing factor in developing a BCC.
Any nonhealing, new-growth lesions on sun-exposed areas should be evaluated for possible nonmelanoma skin cancers such as BCC.
Cryotherapy and topical agents (e.g., imiquimod, 5-fluorouracil) are effective treatments for superficial BCC without the need to undergo invasive surgical procedures.
SQUAMOUS CELL CARCINOMA
I. OVERVIEW
SCC is the second most common form of skin cancer. SCCs frequently develop on sun-exposed areas due to UV irradiation-induced skin keratinocytes damage and malignant transformation. Unlike BCC, SCC may metastasize via lymphatic or hematogenous spread.
A. Definition: SCC is a malignant epithelial neoplasm arising from the mutated keratinocytes in the epidermal layer of the skin.
B. Incidence:
1. Approximately 20% of NMSC occurrence and over 700,000 case annually
2. Higher incidence among men, advanced age, history of excessive sun exposure, and having multiple AKs
3. Risk factors:
a. Chronic sun exposure
b. Immunosuppression
c. Genetic predispositions
d. Chemical carcinogen exposures
e. X-ray irradiation
f. Geographic factors
g. Chronic inflammation/nonhealing wounds
C. Etiology:
1. Cumulated dose of UV radiation: excessive UV light absorbed by DNA of keratinocytes results in damage and malignant transformation. Chronic UVB radiation including excessive childhood sun exposure is the most contributory cause of sun damage in cumulative sun exposure.
2. Ionizing radiation.
3. Scar including thermal injury.
4. Chronic inflammation: nonhealing wounds (e.g., chronic ulcer, burns, and infection) and chronic inflammatory dermatoses such as lichen sclerosus et atrophicus. Cutaneous SCC arising from a chronic wound is also known as Marjolin ulcer.
5. Immunosuppression: solid organ transplant recipients, immunosuppressant medication users, HIV infection, and prolonged use of oral prednisone.
6. Genetic disorders: XP, albinism, epidermolysis bullosa (EB) syndrome, epidermodysplasia verruciformis, Fanconi anemia, Fergus-Smith syndrome, dyskeratosis congenital, Rothmund-Thomson syndrome, Bloom syndrome, and Werner syndrome.
7. Human papillomavirus (HPV) infection: HPV-16, HPV-18, HPV-31, HPV-5, and HPV-8. Large group of HPV are DNA tumor viruses that affect the mucosa and skin epithelial layer, resulting in hyperproliferative lesions and development of SCCs.
8. Organic hydrocarbons (coal, tar, pitch, crude, paraffin oil, lubricating oil, fuel oil, anthracene oil, and creosote) and inorganic arsenic. Presence of palmoplantar arsenical keratoses is the clue of arsenic exposure.
9. PUVA: risk increases with number of treatments, previous exposure of ionizing radiation, and history of prior skin cancers.
10. Use of photosensitizing medications: voriconazole, oral contraceptives, and BRAF inhibitors (vemurafenib, dabrafenib)
11. Precursor lesions include solar keratoses, arsenical keratoses, thermal keratoses, chronic radiation keratoses, tar keratoses, chronic cicatrix keratoses, Bowen disease, erythroplasia of Queyrat, and EV (Lewandowsky-Lutz syndrome).
D. Pathogenesis:
1. Chronic UV exposure induces several different cellular responses, including the induction of stress protein, DNA damage repair process, and cytokine production.
2. In dose-dependent DNA damage, affected cells first either undergo apoptosis (cell death as sunburn cells) or cease proliferating (cell cycle arrest) in order to undergo genetic repair process. Then, hyperproliferation and epidermal thickening follow the growth arrest.
 FIGURE 16-8. Invasive squamous cell carcinoma. (From Weisel, S. W. (2013). Operative techniques in orthopaedic surgery. Philadelphia, PA: Wolters Kluwer.) |
3. In SCC development, accumulated dose of UV radiation exposure induces DNA damage and manifestation of p53 genetic mutation.
4. Mutated p53 in basal layer becomes resistant to UV-induced apoptosis and continuously increases the number of mutated p53 in clonal expansion.
5. Continuous UV irradiation causes second p53 mutation and gains the growth advantage in squamous cell dysplasia in the epidermal layer.
6. Additional genetic alteration leads to proliferation of neoplastic clone and results in SCC in situ at the epidermal surface.
7. Neoplastic tumor progresses to develop invasive property by additional genetic alteration and acquisition of invasive capability to become invasive SCC.
8. Highly differentiated tumors in invasive SCC form epidermal keratinization and invade to the dermis with a broad tumor margin.
 FIGURE 16-9. Nodular squamous cell carcinoma. (From Craft, N., et al. (2010). VisualDx: Essential adult dermatology. Philadelphia, PA: Wolters Kluwer.) |
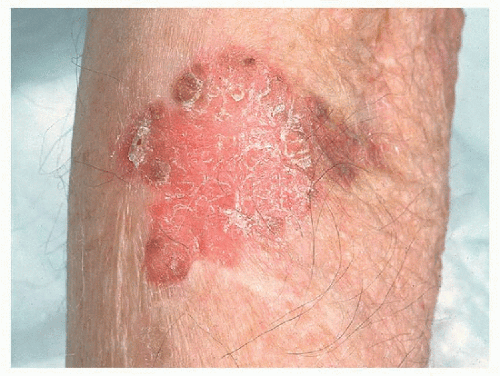 FIGURE 16-10. Squamous cell carcinoma in situ. (From Craft, N., et al. (2010). VisualDx: Essential adult dermatology. Philadelphia, PA: Wolters Kluwer.) |
9. Continuous progression in tumorigenesis with genetic alteration allows the acquisition of the metastatic capacity in the metastasis of SCC.
II. CLINICAL VARIANTS
A. Invasive SCC (Figure 16-8): dysplastic or malignant keratinocytes involve the full thickness of the epidermis and infiltrate through the basal layer, dermis, or deeper tissues/organs.
1. Well differentiated: pink, violaceous, or erythematous; firm, indurated papulonodule or plaque, with hyperkeratotic scale
2. Poorly differentiated: erythematous or pale pink, soft granulomatous papule or plaque may have ulceration, necrosis, or hemorrhaging without hyperkeratosis.
B. Nodular SCC (Figure 16-9): often well-differentiated, pink or erythematous nodule with keratotic scale.
C. SCC in situ (Bowen disease) (Figure 16-10): erythematous, crusty, rough, and scaly patch or slightly raised plaque.
 FIGURE 16-11. Oral squamous cell carcinoma. (From DeLong, L., & Burkhart, N. (2007). General and oral pathology for the dental hygienist. Philadelphia, PA: Lippincott Williams & Wilkins.) |
 FIGURE 16-12. Penile squamous cell carcinoma in situ. (From Craft, N., et al. (2010). VisualDx: Essential adult dermatology. Philadelphia, PA: Wolters Kluwer.) |
D. Oral SCC (Figure 16-11): pink or pale indurated plaque, ulcer, or nodule developed on the floor of the mouth or on the ventral/lateral side of the tongue. SCC may arise from erythroplakia (premalignant erythematous patches) or leukoplakia (persistent white plaques) of the oral mucosa.
E. Erythroplasia of Queyrat (SCC in situ of the glans penis) (Figure 16-12): erythematous, well-demarcated velvety patch or slightly raised plaque.
F. Keratoacanthoma (Figure 16-13): fast-growing (1.5 to 2 cm in 4 to 6 weeks), erythematous, pink, or flesh-colored papule or nodule with central keratotic plug.
G. Verrucous carcinoma (Figure 16-14): indolent form of a well-defined, exophytic, large wart-like papillomatous growth involving oral mucosa (oral florid papillomatosis), anogenital areas (condyloma acuminatum of Buschke-Lowenstein), and plantar foot (epithelioma cuniculatum).
 FIGURE 16-13. Keratoacanthoma. (From Goodheart, H. P. (2003). Goodheart’s photoguide of common skin disorders (2nd ed.). Philadelphia, PA: Lippincott Williams & Wilkins.) |
III. LABORATORY AND DIAGNOSTIC TESTS
A. Clinical evaluation and skin biopsy for confirmation of diagnosis
B. Computed tomography (CT) scanning evaluation for possible metastasis to nearby bones and soft tissues for aggressively developing invasive-type SCC lesions
C. Magnetic resonance imaging (MRI) evaluation for possible involvement of perineural, orbital, or intracranial nerves for neurologic symptoms (local paresthesia, numbness, pain, visual changes, etc.)
IV. DIFFERENTIAL DIAGNOSIS
A. Amelanotic melanoma
B. Basal cell carcinoma
C. Hyperkeratotic actinic keratosis
D. Inflamed seborrheic keratosis
E. Merkel cell carcinoma
F. Nummular eczema
G. Paget disease
H. Prurigo nodularis
I. Psoriasis
J. Pyogenic granuloma
K. Traumatic ulcer/chronic inflammation
L. Viral warts (verruca)
V. TREATMENT MODALITIES
A. Mohs micrographic surgery: a treatment of choice for invasive SCC and high-risk SCC. Mohs procedure is particularly preferred for cosmetically sensitive areas on the head and neck.
1. Histopathologic evaluations of entire margins and depth of the specimen are performed during the procedure (repeated process of harvesting specimen from the surgical defect until total clearance).
2. Cure rate for tumors smaller than 2 cm is 98%; decreased cure rate to 75% for tumors larger than 2 cm.
B. Conventional excision surgery: a typical treatment method for tumors smaller than 2 cm, low-grade, or well-differentiated type on the trunk and extremities.
C. Electrodessication and curettage: treatment for low-grade, small, superficial, and low-risk tumors, preferred on the trunk and extremities.
1. Less invasive treatment option compared to conventional excision surgery
2. Useful treatment option for patients in advanced age and poor surgical candidates
D. Cryotherapy: may be effective in treating very small, lowrisk, superficial tumors.
E. 5-Fluorouracil (5-FU) and imiquimod topical treatments (biologic modifiers): effective in treating superficial type and early-stage SCC lesions progressing from AK.
F. PDT using photosensitizing agents with blue light (wavelength 400 nm) is effective in destroying skin cancer cells.
G. Radiation therapy is used in combination with other treatment modalities for large tumors; aggressive, recurrent, or inoperative cases; and poor candidates for invasive surgeries.
H. Regional control
1. Nonpalpable nodes
a. Close monitoring for lymphadenopathy
b. Sentinel node biopsy for high-risk SCCs, followed by elective lymph node dissection if node is positive
c. Radiation to draining (primary echelon) nodes for high-risk lesions
2. Palpable nodes
a. Radiation
b. Surgery
c. Chemotherapy
d. Combination of above
VI. PROGNOSIS
The risk of metastasis and prognosis for SCC depend on the size of the tumor, location, depth, perineural involvement, and immunosuppression. SCC lesions developed from ulcerated wounds, chronic inflammation, and recurrent cutaneous lesions are considered high risk for metastasis.
Squamous Cell Carcinoma
History of chronic UV radiation exposure, including life style, occupational and recreational sun exposure, is the main contributing factor of developing SCC.
Contact with carcinogenic and arsenic chemicals may contribute to development of SCC in situ (Bowen disease).
SCC occurs most commonly on the head, neck, and arms.
Chronically inflamed, ulcerated, and nonhealing wounds should be considered for a skin biopsy to exclude SCC (Marjolin ulcer).
Regular practice of photoprotection (e.g., wearing sun protective clothing, sunglasses, and sunscreen topicals) and seeking treatments for precancerous lesions (AKs) may lead to early detection of SCC and effectively prevent further skin sun damage.
KERATOACANTHOMA
I. OVERVIEW
Keratoacanthoma (KA) is a low-grade, well-differentiated variant of SCC, while the exact classification of the disease is still uncertain and some consider it a benign tumor. KA develops commonly on sun-exposed areas (e.g., head, neck, and extremities). It grows rapidly in 2 to 6 weeks to reach the average size of 0.5 to 2.5 cm in diameter, but it is rarely metastasized. It is manifested as a volcano-like dome-shaped plaque or an exophytic sharply circumscribed, crateriform nodule with central keratotic plug or scale. KA regresses in weeks to months if it is left untreated; however, the regression may result in an irregularly shaped atrophic scar. KA resembles SCC clinically as well as microscopically and is very difficult to distinguish from an invasive SCC.
A. Definition: KA is a rapidly proliferating, highly differentiated tumor of squamous epithelia, with a keratin scale or keratotic plug, arising from the neck of the hair follicle.
B. Etiology: the origin of the KA is still unclear. Below are the proposed etiologies of KA:
1. Exposure to carcinogenic chemicals such as tar pits.
2. Smoking
3. Immunosuppression
4. HPV infection
5. Associating with Muir-Torre syndrome (defective DNA mismatch repair gene)
C. Pathogenesis:
1. Some case studies report KA arises from p53 mutation and overexpression of P53 protein in signaling pathway.
2. Microsatellite instability and mismatch repair deficient in genetic defect may have association with cancerprone Muir-Torre syndrome.
II. CLINICAL VARIANTS
Clinical presentation of KA resembles SCC, even at the microscopic level; therefore, the differentiation between SCC and KA can be challenging.
A. Clinical stages in KA manifestation:
1. Proliferative stage: sudden development and growth of firm papule with fine telangiectases for 2 to 4 weeks
2. Mature stage: formation of a nodular plaque with central keratotic scale or plug
3. Resolving/involution stage: tumor reabsorption within 4 to 6 months, result in slightly atrophic, depressed, and pale scar
1. KA centrifugum: solitary lesion that sizes up to 20 cm with simultaneous healing at the center of the lesion. Common sites are on the face, trunk, or extremities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






