Chemotherapy
Chemotherapy is the use of one or more drugs to destroy cancer cells or suppress their growth. A single drug typically gives a limited response, but if two or more drugs are used, long remissions or cures may occur. Unfortunately, remissions and cures aren’t always possible. That’s because many cancers become resistant to chemotherapeutic drugs. Also, the toxic effects of some drugs may prevent dosages high enough to effectively destroy the cancer cells. (See How chemotherapeutic drugs disrupt the cell cycle.)
Because many types of cancer exist, various types and combinations of chemotherapeutic drugs must be used. Classified according to mechanism of action, these drugs include alkylating agents, antimetabolites, antibiotic antineoplastic agents, and hormonal antineoplastic agents.
Alkylating agents can inhibit cell division at any point in the cell cycle, but they’re particularly effective in the late G1 and S phases. Examples of alkylating agents include busulfan, carmustine (BCNU), cisplatin, ifosfamide, and mechlorethamine. Given alone or with other drugs, they help treat chronic and acute leukemias, non-Hodgkin’s lymphomas,
multiple myeloma, melanoma, sarcoma, and cancers of the breast, ovaries, uterus, lung, brain, testes, bladder, prostate, and stomach.
multiple myeloma, melanoma, sarcoma, and cancers of the breast, ovaries, uterus, lung, brain, testes, bladder, prostate, and stomach.
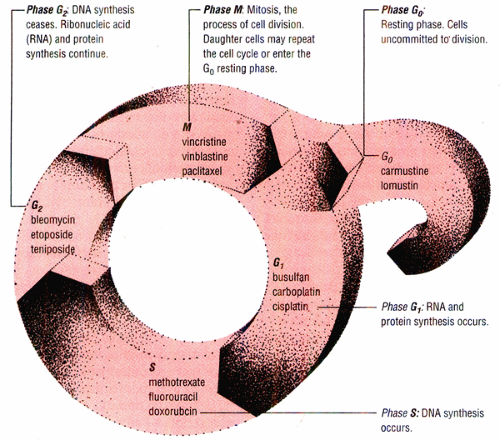
How chemotherapeutic drugs disrupt the cell cycle
Chemotherapeutic drugs may be either cell-cycle specific or cell-cycle nonspecific. Cell-cycle-specific drugs act at one or more cell-cycle phases. Cell-cycle-nonspecific drugs can act on both replicating and resting cells.
Note: The drugs listed in this diagram are only examples of cell-cycle-specific agents
Antimetabolites structurally resemble natural metabolites. As a result, they can become involved in processes associated with the natural metabolites—that is, the synthesis of nucleic acids and proteins. However, the antimetabolites differ sufficiently from the natural metabolites to interfere with this synthesis. Because the antimetabolites are cell-cycle specific and primarily affect cells that actively synthesize deoxyribonucleic acid (DNA), they are referred to as S-phase specific. Examples of antimetabolites include mercaptopurine and thioguanine, which inhibit purine synthesis; cytarabine, floxuridine, and fluorouracil, which inhibit pyrimidine synthesis; and methotrexate, which prevents reduction of folic acid to dihydrofolate reductase. Cancers that respond to the action of antimetabolites include acute leukemia, breast cancer, adenocarcinomas of the GI tract, non-Hodgkin’s lymphomas, and squamous cell carcinomas of the head, neck, and cervix.
Antibiotic antineoplastic agents achieve their effects by binding with DNA. These drugs inhibit the cellular processes of normal and malignant cells and are cell-cycle nonspecific, except for bleomycin, which causes its major effects in the G2 phase of the cell cycle. Examples of antibiotic antineoplastics include bleomycin, dactinomycin, doxorubicin, and mitomycin, which are used mainly to treat carcinomas, sarcomas, and lymphomas; daunorubicin, which is used in acute nonlymphoblastic leukemia; and mitoxantrone, which is used to treat acute nonlymphocytic leukemia and breast cancer.
Hormonal antineoplastic agents inhibit cancer growth in specific tissues without directly causing toxicity. Although the mechanisms of action are not completely understood, hormonal therapies prove effective against hormone-dependent tumors, such as cancers of the prostate, breast, and endometrium. Some examples of hormonal antineoplastic agents include estrogens (such as chlorotrianisene and diethylstilbestrol), antiestrogens (such as tamoxifen citrate), androgens (such as testolactone and testosterone), progestins (such as medroxyprogesterone acetate), corticosteroids (such as prednisone and dexamethasone), and gonadotropinreleasing hormone analogues (such as leuprolide acetate).
Tubulin-interactive agents, such as the vinca alkaloids (vincristine and vinblastine) and paclitaxel, interfere with normal microtubule function necessary for mitosis. Vinca alkaloids are used to treat lymphomas, leukemias, and sarcomas, whereas paclitaxel is used in breast and ovarian cancers.
A chemotherapeutic regimen may consist of one or multiple drugs. It may also be used alone or as an adjunct to surgery or radiation therapy.
Procedure
Chemotherapy may be administered in a health care facility, an ambulatory care setting, or the patient’s home. The drugs are typically given intermittently to allow healthy tissues time to recover and to minimize adverse reactions. Chemotherapy may be given by any one of a number of routes, including I.M., I.V., subcutaneous, intra-arterially, orally, topically, intracavitarily, intravesically, and intrathecally.
Of these routes, the I.V. route is the most common for administering chemo-therapeutic drugs because, whether given through a peripheral or central line, it delivers the drug directly to the body’s tissues. On occasion, a patient may require continuous chemotherapy, in which case an ambulatory infusion pump may be used.
Only trained personnel, using a laminar airflow hood, should reconstitute chemotherapeutic drugs. Furthermore, only physicians or chemotherapy-certified nurses should administer the drugs. The route, dose, frequency, and length of administration of each drug will vary according to the type of cancer and the patient’s physical condition.
Complications
The adverse effects of chemotherapy depend on the specific drug used, its dose and administration schedule, the type and extent of the cancer, the patient’s physical and psychological status, and the patient’s sensitivity to the drug. Some patients experience only mild or temporary reactions, whereas other patients experience severe or even permanent effects.
The most common—and potentially most serious—adverse reaction is bone marrow suppression (myelosuppression). This reaction causes the number of circulating blood cells to drop, placing the patient at risk for several problems. A reduced number of white blood cells (leukopenia) increases the patient’s risk of infection, especially if the granulocyte count is under 1,000/mm3; a low platelet count (thrombocytopenia) increases the patient’s risk of bleeding; and a fall in red blood cells can lead to anemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree