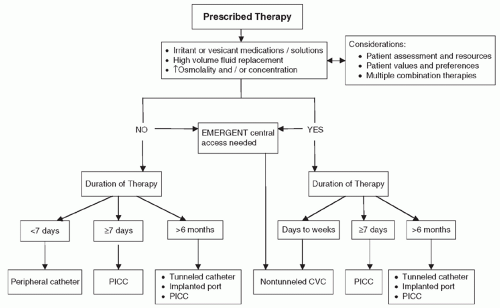
or incompatible solutions. Another advantage of CVADs is that they may stay in place for weeks to years, depending on the type of CVAD.
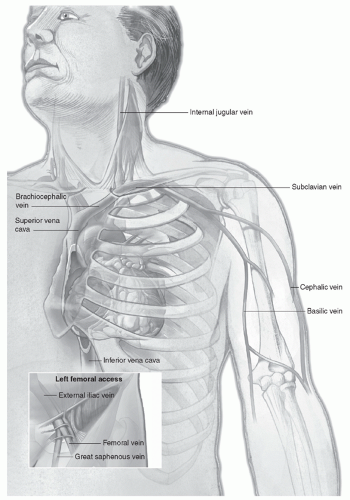
At the angle of junction, the left subclavian receives the thoracic duct, whereas the right subclavian receives the right lymphatic duct.
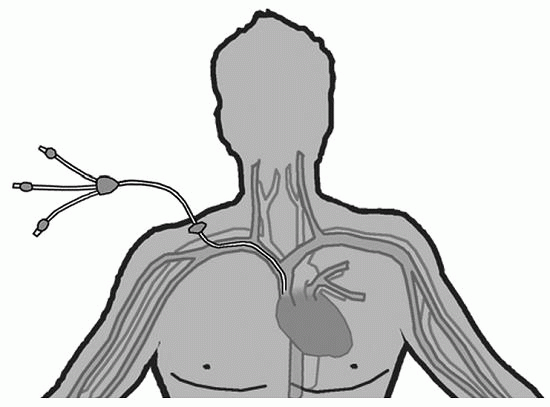
Nontunneled CVAD |
|
Nontunneled CVAD |
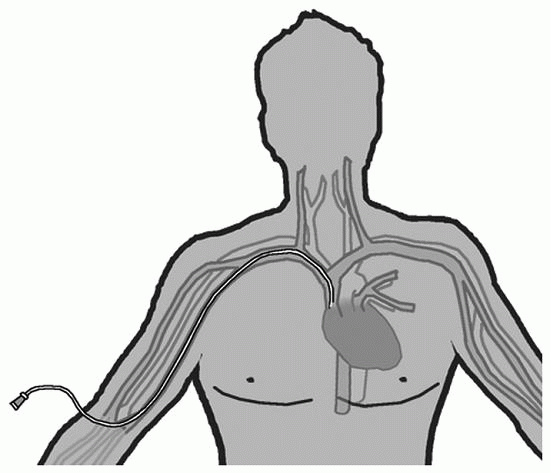
PICC |
|
PICC |
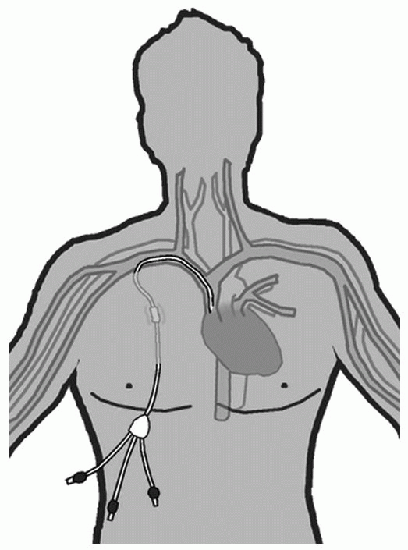
Tunneled CVADs |
|
Tunneled CVAD |
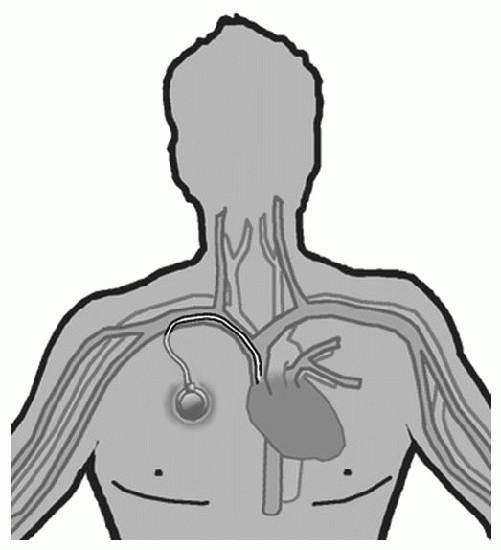
Implanted Port |
|
Implanted port |
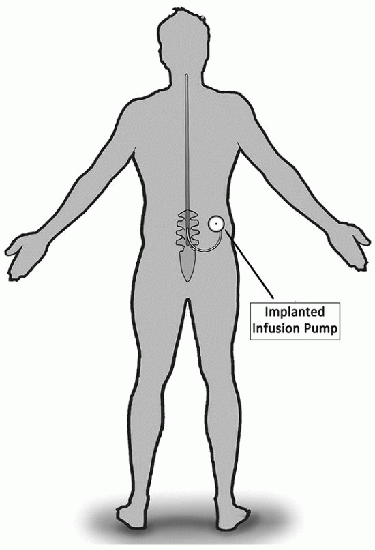
Implanted Infusion Pump |
|
Implanted infusion pump |
Decreased risk of bleeding or air emboli
Elimination of catheter clamping
Elimination of the use of heparin in the catheter
Reduction of flushing between use; weekly flushing is all that is required
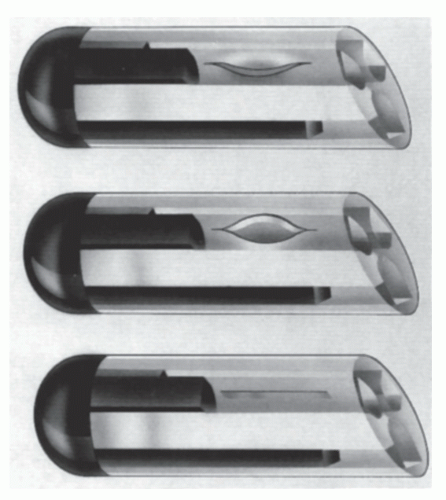
opens with minimal positive pressure for infusion, yet requires four times as much negative pressure for aspiration. The PASV also reduces the reflux of blood into the end of the catheter during periods of increased CVP and decreases difficulties with poor blood return. Weekly saline-only flushing is needed, and the product is consistent with needleless systems. The valve may also be used with PICCs and midline catheters. It is important to note that changes in technique are needed when this type of catheter and valve are used. The catheter must not be clamped until after the syringe is removed or the valve will not function to prevent reflux. When the catheter is not in use, the catheter hub can be taped to the chest wall above heart level. This minimizes blood pressure at the catheter tip.
health care providers and nurses inserting and maintaining CVADs as mentioned earlier (CDC, 2011; INS, 2011). Grouping key actions to prevent CRBSIs has been referred to as the Central Line Bundle (CDC, 2011; IHI, 2011; INS, 2011). These measures include optimal catheter and site selection (avoiding the femoral vein for a CVAD in an adult), appropriate hand hygiene (should be practiced before and after insertion site palpation, before and after insertion, and before and after donning and removing gloves), the use of chlorhexidine gluconate solution as a skin antiseptic, and maximal barrier precautions during insertion of the CVAD. This requires the use of a mask, cap, sterile gown, and sterile gloves for the health care provider and a sterile full body drape for the patient (CDC, 2011; IHI, 2011). Daily assessment of the need for the CVAD and prompt removal when indicated prevents infection (CDC, 2011). The Agency for Healthcare Research and Quality (AHRQ) developed a toolkit for preventing CRBSI; a daily audit form illustrates the best practices that should be followed (Figure 14-5). Reports of agencies implementing these actions and the reduction in adverse events, or the outcomes of monitoring adherence to procedures, all demonstrate the success of these interventions (Guerin, Wagner, Rains, & Bessesen, 2010; The Joint Commission, 2012; McMullan et al., 2013; Rupp et al., 2013).
 FIGURE 14-5 AHRQ CVC Maintenance Audit Form. (From Agency for Healthcare Research and Quality. [2013].) |
 PATIENT SAFETY
PATIENT SAFETYinfusion pump is being inserted, offer the patient the opportunity to see and/or handle a sample of the CVAD that will be placed. This often helps to reassure many patients. Explain to the patient that he or she will be covered with a sterile drape, and the health care providers will wear gowns, caps, masks, and gloves.
Assessment of patient’s readiness for learning, potential barriers to learninghealth literacy, and preferred learning style(s)
Rationale for CVAD placement and device options including length of therapy and impact on patient’s lifestyle
Discussion of the insertion procedure and maintenance of the CVAD: Include patient and health care provider responsibilities for the maintenance of the CVAD once inserted
Explanation of possible risks and complications of CVAD insertion and continued CVAD utilization
Emergency information includes what to do and whom to call if the external catheter is cut, breaks, cracks, or is accidently removed
When to return for follow-up appointments for assessment and maintenance of the CVAD
Use of multiple methods of instruction including written materials, multimedia presentations, hands-on opportunities, and interactive learning whenever possible
Assessment of patient and/or caregiver comprehension through discussion and return demonstration
Opportunities for patient and caregiver questions and/or concerns
Abnormal coagulation studies
Septicemia
Anomalies of the central venous vascular structures
Thrombosis of the subclavian or brachiocephalic veins or the superior vena cava
 EVIDENCE FOR PRACTICE
EVIDENCE FOR PRACTICE
Central line bundle, consisting of hand hygiene, maximum barrier precautions, chlorhexidine site disinfection, avoiding the femoral site, and promptly removing unnecessary CVCs
Procedure checklist to ensure adherence to proper practices
Process permitting a team member to stop the CVC insertion in nonemergent situations if evidence-based practices are not being followed
Radiation skin damage at intended insertion site
Fractured clavicle
Hyperinflated lungs
Malignant lesion at the base of the neck or apex of the lungs
the lower pair is located at the vein’s entrance to the subclavian vein. Because of these factors, central catheterization can be difficult. Because a short catheter may be inserted easily, central catheterization may be achieved by using an introducer with a guidewire. Entry into the superficial vein is performed by directing the catheter toward the ipsilateral nipple.
Catheter occlusion, resulting from the patient’s head movement, is a persistent problem.
Vein irritation also results from head movement, with a shorter catheter life as a consequence.
Maintaining an intact dressing on the area is difficult.
The idea of having a catheter in the neck may be aesthetically and psychologically disturbing to many patients and families.
 PATIENT SAFETY
PATIENT SAFETYTABLE 14-1 POTENTIAL COMPLICATIONS OF CVC INSERTION OR CVADs | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
May be inserted at the bedside or in radiology under fluoroscopy by either RNs or physicians/LIPs
Decreased risk of insertion-related complications compared with other CVADs
Lower infection rates than other CVADs
Better suited for young children or the elderly because of the small gauge
Cost-effective when compared with surgically placed CVADs
blood sampling should not be taken from the arm with a PICC insertion. The presence of any of the following conditions may be a contraindication for a PICC in that extremity:
TABLE 14-2 CVAD DESCRIPTIONS, INSERTION TECHNIQUES, AND CONSIDERATIONS | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree