Antigen—protein that stimulates an immune reaction, causing the production of antibodies.
Antibody—globulin (protein) secreted by B cells as a defense mechanism against foreign materials.
Atopy—term that refers to the ability to produce immunoglobulin E antibodies to common allergens.
Immunity:
Humoral—process by which B lymphocytes produce circulating antibodies to act against antigens.
Cell-mediated—portion of the immune system in which the participation of T lymphocytes and macrophages is predominant.
Mast cell—tissue cell similar to peripheral blood basophil, which contains granules with chemical mediators.
Basophil—leukocyte with large granules that contain histamine.
Hypersensitivity—reaction to an antigen after reexposure (there are four types); type I (immediate) and type IV (delayed) are considered allergic reactions.
The abbreviation for immunoglobulin is Ig.
Antibodies combine with antigens in lock-and-key style.
There are five major classes of immunoglobulins.
IgM—constitutes 10% of immunoglobulin pool; found mostly in intravascular fluid and primarily engaged in initial defense; levels elevated with recent infection.
IgG—major immunoglobulin that accounts for 70% to 75% of secondary immune responses and combats tissue infection.
IgA—15% to 20% of immunoglobulins; predominantly found in seromucous secretions (such as saliva, tears) in which it provides a primary defense mechanism.
IgD—less than 1% of immunoglobulin pool; found on circulating B lymphocytes, and signals B cells to become activated.
IgE—only a trace found in serum; attaches to surface membrane of basophils and mast cells; responsible for immediate types of allergic reactions.
Characterized by:
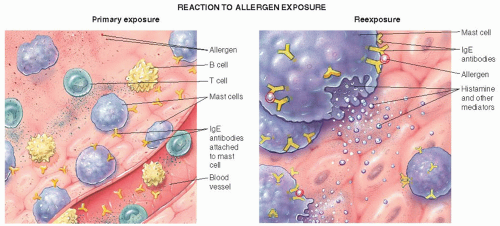
IgE-mediated allergic reaction (see Figure 28-1).
Occurs immediately after contact with the antigen.
Causes release and neo-synthesis of preformed chemical mediators.
Examples—anaphylaxis, allergic rhinitis, urticaria.
Histamine—bioactive amine stored in granules of mast cells and basophils.
Leukotrienes—newly synthesized potent bronchoconstrictors; cause increased venous permeability.
Prostaglandins—potent vasodilators and potent bronchoconstrictors.
Platelet-activating factor—has many properties; causes the aggregation of platelets.
Cytokines—control and regulate immunologic functions (eg, interleukins, tumor necrosis factor).
Proteases—enzymes, such as tryptase and chymase, increase vascular permeability.
Eosinophil chemotactic factor of anaphylaxis—causes an influx of eosinophils into the area of allergic inflammation.
Generalized vasodilation, hypotension, flushing.
Increased permeability.
Capillaries of the skin—edema.
Mucous membranes—edema.
Smooth muscle contraction.
Bronchioles—bronchospasm.
Intestines—abdominal cramps, diarrhea.
Increased secretions.
Nasal mucous glands—rhinorrhea.
Bronchioles—increased mucus in airways.
GI—increased gastric secretions.
Lacrimal—tearing.
Salivary—salivation.
Pruritus (itching).
Skin.
Mucous membrane.
Characterized by a cell-mediated reaction between antigens and antigen-responsive T lymphocytes.
Maximal intensity occurs between 24 and 48 hours.
Usually consists of erythema and induration.
Examples—tuberculin skin test; contact dermatitis such as poison ivy.
Evaluate for symptoms related to hay fever, asthma, skin reactions, insect allergy, and food allergy.
Determine exacerbating factors such as contact with pets, outdoor exposure, a certain season, contact with mold, exposure to dust.
Obtain complete medical history for past illnesses, medication allergies, family history, medications that have been tried, exercise, smoking, and work environment.
Perform physical examination based on patient presentation and specific allergy condition, usually skin, head, chest, eye, ear, nose, and throat examination.
Advantages:
Efficient—results within 15 minutes.
Little discomfort to the patient.
Only rare instances of anaphylaxis because of minimal systemic absorption.
Disadvantages:
Old or thick, leathery skin decreases reactivity.
Drops have a tendency to run together, which would affect the accuracy of the test.
Advantages:
Useful to confirm equivocal epicutaneous results with some antigens.
Disadvantages:
Less specific than prick testing.
Increased possibility for anaphylactic reactions.
Requires more time and skill to perform.
Increased discomfort to the patient.
In vitro—using blood samples—tests for IgE antibodies to specific allergens. Instead of looking for a reaction in vivo (with the patient’s body—as in skin testing), in vitro testing measures the IgE response to specific antigens added to blood samples. Advantages over skin testing include the following:
Can be done without special knowledge of skin testing or availability of allergen extract.
Patient does not need to stop antihistamine before testing.
Can be done even with severe eczema.
There is no risk of systemic reaction.
An immunofluorescent process is preferred for specific IgE testing because of high degree of sensitivity and specificity.
Some labs may offer a radioallergosorbent test (RAST); measures the allergen-specific IgE antibodies in serum samples after panel of allergens have been added to samples.
Tell the patient that it is allergy testing without the risk of causing severe allergic reaction.
Obtain adequate venous blood for each allergen panel to be tested.
A positive result depends on the standards for that particular laboratory. The test does not indicate clinical significance of symptoms and must be interpreted with patient’s history.
Arrange for a follow-up visit for the patient with the health care provider to discuss test results.
 Evidence Base
Evidence Base
Specific allergens are identified by skin or blood testing.
Serial injections are begun that contain extracts from identified allergens (allergy vaccine).
Initially, a small amount of dilute allergy vaccine is given, usually at weekly intervals.
Amount and concentration are slowly increased to maximum tolerable dose.
The maintenance dose is injected every 2 to 4 weeks for a period of several years to achieve maximal benefit.
Several allergens are now standardized (dust mite, cat, grass and ragweed pollens, Hymenoptera venoms [yellow jacket, yellow and white-faced hornet, honey bee, wasp]).
Anaphylaxis rarely occurs after injection but risk remains.
Should be given only in health care facility with epinephrine, trained personnel, and emergency equipment available. See Standards of Care Guidelines 28-1, page 1026.
Patient should remain in office for 30 minutes after injection, after which the risk of anaphylaxis is greatly reduced.
If large, local reaction (erythema, induration) occurs after an injection, the next dose should not be increased without checking with prescribing health care provider because a systemic reaction may occur.
If several weeks are missed, dosage may need to be decreased to prevent a reaction.
Medication, such as antihistamines and decongestants, should be continued until significant symptom relief occurs (may take 12 to 24 months).
Environmental controls should be maintained to enhance effectiveness of therapy.
| ||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 NURSING ALERT
NURSING ALERT
| |||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
May be caused by:
Immunotherapy.
Stinging insects.
Skin testing.
Medications.
Contrast media infusion.
Foods.
Exercise.
Latex.
Release of chemical mediators results in massive vasodilation, increased capillary permeability, bronchoconstriction, and decreased peristalsis.
Make sure that the patient remains under observation for at least 30 minutes after injection and assess for local reaction and respiratory distress before the patient leaves.
If a significant reaction occurred after the last injection or if the patient is late for his or her dosage, follow written protocol for those cases or call the patient’s health care provider for instructions.
Be prepared to inject 0.3-0.5 mL (or 0.01 mL/kg for children <30 kg) of epinephrine I.M. as directed by health care provider present or facility protocol if signs of anaphylaxis develop. Call for immediate help and transfer to an acute care facility.
Respiratory—laryngeal edema, bronchospasm, cough, wheezing, dyspnea, lump in throat.
Cardiovascular—hypotension, tachycardia, palpitations, syncope.
Cutaneous—urticaria (hives), angioedema, pruritus, erythema (flushing).
GI—nausea, vomiting, diarrhea, abdominal pain, bloating.
 DRUG ALERT
DRUG ALERT
Place patient supine and check vital signs.
Immediately administer epinephrine 1:1,000—adolescents and adults, 0.3 to 0.5 mL; children, 0.01 mL/kg via intramuscular (I.M.) route into vastus lateralis muscle. This may be repeated every 5 to 10 minutes if necessary—causes vasoconstriction, decreases capillary permeability, relaxes airway smooth muscle, and inhibits mast cell mediator release.
Monitor vital signs continuously. Administer oxygen, if needed.
A tourniquet is applied above site of antigen injection (allergy injection, insect sting, etc.) or skin test site to slow the absorption of antigen into the system.
An adequate airway is established and albuterol is administered by inhalation, as needed.
Hypotension and shock are treated with fluids and vasopressors.
Additional bronchodilators are given to relax bronchial smooth muscle.
Histamine-1 (H1) antihistamines, such as diphenhydramine, and, possibly, H2 antihistamines, such as ranitidine, are given to block the effects of histamine.
Corticosteroids are given to decrease vascular permeability and diminish the migration of inflammatory cells; may be helpful in preventing late-phase responses.
Cardiovascular collapse.
Respiratory failure.
Promptly assess airway, breathing, and circulation (ABCs) with severe presentation and intervene with cardiopulmonary resuscitation, as appropriate.
When ABCs are stable, assess vital signs, degree of respiratory distress, and angioedema.
Obtain a history of onset of symptoms and of exposure to allergen.
Ineffective Breathing Pattern related to bronchospasm and laryngeal edema.
Decreased Cardiac Output related to vasodilation.
Anxiety related to respiratory distress and life-threatening situation.
Establish and maintain an adequate airway.
If epinephrine has not stabilized bronchospasm, assist with endotracheal intubation, emergency tracheostomy, or cricothyroidotomy, as indicated.
Continually monitor respiratory rate, depth, and breath sounds for decreased work of breathing and effective ventilation.
Administer nebulized albuterol or other bronchodilators, as ordered. Monitor heart rate (increased with bronchodilators).
Provide oxygen via nasal cannula at 2 to 5 L/minute or by alternative means, as ordered.
Administer intravenous (IV) corticosteroids, as ordered.
Monitor blood pressure (BP) by continuous automatic cuff, if available.
Administer rapid infusion of IV fluids to fill vasodilated circulatory system and raise BP.
Monitor central venous pressure (CVP) to ensure adequate fluid volume and to prevent fluid overload.
Insert indwelling catheter and monitor urine output hourly to ensure kidney perfusion.
Initiate and titrate vasopressor, as ordered, based on BP response.
Provide care in a prompt, calm, and confident manner.
Remain responsive to the patient, who may remain alert but not completely coherent because of hypotension, hypoxemia, and effects of medication.
Keep family or significant others informed of patient’s condition and the treatment being given.
When patient is stable and alert, give a simple, honest explanation of anaphylaxis and the treatment that was given.
Make sure that patient who has experienced anaphylaxis or severe local reactions obtains a prescription for self-injectable epinephrine to have available at all times.
Instruct the patient and family members in the injection technique upon exposure to known antigen or at the first signs of a systemic reaction.
Provide patient with information on epinephrine, including dose, drug action, possible adverse effects, the importance of prompt administration at the first sign of a systemic reaction, storage conditions, and replacement of outdated syringe.
Ensure day care providers and school personnel are aware of patient’s potential for anaphylaxis and have access to and are able to administer epinephrine.
Even if treatment is given successfully at home, the patient should follow up with the health care provider immediately.
Make sure that the patient with history of anaphylaxis has access to emergency medical system and does not spend time alone if risk of reaction is present.
Teach the patient at risk for anaphylaxis about the potential seriousness of these reactions.
Educate patient to recognize the early signs and symptoms of anaphylaxis and have epinephrine available.
People allergic to venom stings should avoid wearing brightly colored or black clothes, perfumes, and hair spray. Shoes should be worn at all times.
For exercise-induced anaphylaxis, patient should exercise in moderation, preferably with another person, and in a controlled setting, where assistance is readily available.
Instruct patient to wear a MedicAlert-type bracelet at all times.
For potential drug allergies, teach the patient to:
Read labels and be familiar with the generic name of the drug thought to cause a reaction.
Discard all unused drugs. Make sure any drug kept in the medicine cabinet is clearly labeled.
Become familiar with drugs that may cross-react with a drug to which patient is allergic.
Always know the name of every drug taken.
Clear all herbals and nutraceuticals with health care provider.
Advise patient with a known sensitivity to a food product to be extremely careful about everything he or she eats—allergen compounds may be hidden in a preparation (such as caseinate, lactalbumin).
Advise that if food is associated with exercise-induced anaphylaxis, wait at least 2 hours after eating to exercise.
Respirations unlabored with clear lung fields, minimal wheezing.
BP and CVP within normal range; urine output adequate.
Responsive and cooperative.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



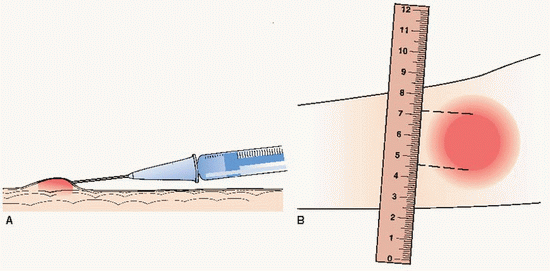 (A) Intradermal injection producing a wheal. (B) Measuring area of induration.
(A) Intradermal injection producing a wheal. (B) Measuring area of induration. Key Decision Point If signs of anaphylaxis develop, stop testing and apply tourniquet above skin testing site. Administer epinephrine I.M. (using vastus lateralis muscle) and administer oxygen. Alert provider or emergency responders.
Key Decision Point If signs of anaphylaxis develop, stop testing and apply tourniquet above skin testing site. Administer epinephrine I.M. (using vastus lateralis muscle) and administer oxygen. Alert provider or emergency responders. Evidence Base Lieberman, P., Nicklas, R. A., Oppenheimer, J., et al. (2010). Diagnosis and management of anaphylaxis practice parameter: 2010 update. Journal of Allergy and Clinical Immunology, 126, 477-480.
Evidence Base Lieberman, P., Nicklas, R. A., Oppenheimer, J., et al. (2010). Diagnosis and management of anaphylaxis practice parameter: 2010 update. Journal of Allergy and Clinical Immunology, 126, 477-480. NURSING ALERT Because of the risk of anaphylaxis, have epinephrine 1:1,000 readily available for injection, as well as oxygen, parenteral diphenhydramine, and IV supplies.
NURSING ALERT Because of the risk of anaphylaxis, have epinephrine 1:1,000 readily available for injection, as well as oxygen, parenteral diphenhydramine, and IV supplies. Key Decision Point If angioedema, respiratory distress, or hypotension develop, institute emergency actions, including administration of oxygen, application of tourniquet above the injection site, and injection of epinephrine 1:1000 0.3-0.5 mL in the opposite arm.
Key Decision Point If angioedema, respiratory distress, or hypotension develop, institute emergency actions, including administration of oxygen, application of tourniquet above the injection site, and injection of epinephrine 1:1000 0.3-0.5 mL in the opposite arm. Evidence Base Joint Task Force on Practice Parameters for Allergy and Immunology. (2010). Allergen immunotherapy: A practice parameter third update. Journal of Allergy and Clinical Immunology.
Evidence Base Joint Task Force on Practice Parameters for Allergy and Immunology. (2010). Allergen immunotherapy: A practice parameter third update. Journal of Allergy and Clinical Immunology.