Antineoplastic and Biologic Therapy
Lynn M. Czaplewski MS, RN, ACNS-BC, CRNI®, AOCNS®
Cora Vizcarra MBA, RN, CRNI®
PART I: ANTINEOPLASTIC THERAPY
 I. Overview
I. OverviewA. Antineoplastic therapy in the treatment of cancer includes the use of chemotherapy agents and biotherapy
B. Chemotherapy agents directly kill cancer cells as well any cells in the body that replicate frequently
C. Biotherapy in the treatment of cancer includes biologic response modifiers, gene therapy, monoclonal antibodies, and anti-angiogenic agents
 II. The Cell Cycle
II. The Cell CycleA. Overview
1. The cell cycle is a series of phases in normal and cancer cell growth resulting in cell division into two daughter cells
2. The knowledge of cell growth and division helps in the understanding of the mechanisms of action and side effects of antineoplastic therapy
B. Cell Cycle of Normal Cells
1. After cell division, the daughter cells either execute the programmed functions of a specific tissue or enter the cell cycle again to reproduce
2. Normal cells in the resting stage reenter the cell cycle and reproduce only when necessary
3. A control mechanism prevents cell replication unless a cell needs to be replaced as a result of damage or death
4. The cell cycle consists of five stages or phases
a. G0: resting stage, in which the cell is not dividing; it may reenter the cell cycle to replicate
b. G1: stage of ribonucleic acid (RNA) and protein synthesis
c. S: stage of deoxyribonucleic acid (DNA) synthesis
d. G2: pre-mitotic stage (manufactures mitotic spindle)
e. M: mitosis stage (actual cell division)
5. Cell cycle time is the length of time needed for a cell to replicate
C. Cancer Cell Development
1. Cancer cells possess characteristics that allow for uncontrolled cell division
2. Cancer cells continue to divide avoiding normal cell death (apoptosis) resulting in tumor growth and spread into surrounding tissues
3. The cancer cell mass is composed of cells that are dividing or resting
a. The percentage of dividing cancer cells is called the “growth fraction”
b. The term “tumor burden” refers to the number of cancer cells in the body
 III. Drug Specificity
III. Drug SpecificityA. Overview
1. Most chemotherapeutic agents exert the greatest kill of cell when cells are actively dividing. Both malignant cells and normal cells are affected.
a. The smaller the cancer cell mass, the more the cell division is occurring
b. The smaller the mass, the more effective the agent
2. Antineoplastic agents cannot distinguish cancer cells from normal cells
a. Normal cells that are frequently dividing are found in the bone marrow, gastrointestinal (GI) tract, mucosa, gonads, and hair follicles
b. Side effects from the agents result from damage to these tissues: myelosuppression, nausea, vomiting, diarrhea, mucositis, infertility, and alopecia
3. The ideal dose of an antineoplastic agent is one that maximizes cell kill without life-threatening toxicities; time is allowed between cycles to allow normal cells to recover
4. Treatment is determined by the type and stage of the cancer as noted in a pathology report and by the patient’s performance status
5. Chemotherapeutic agents are categorized by their effect on the cell cycle
a. Cell cycle-specific agents exert their greatest effect on one or more phases in the cell cycle
b. Cell cycle-nonspecific agents exert their effect on all phases of the cell cycle
B. Cell cycle-specific Agents
1. Act on the cells at various phases of the cell cycle, for example, inhibit mitosis (vinca alkaloids), interfere with DNA synthesis, or prevent cell reproduction (antimetabolites)
2. Most effective when a large number of cells are dividing
C. Cell cycle-nonspecific Agents
1. Act on cells that are not going through the division phase
2. Prevent cell division by causing chromosome breakage and formation of new cellular DNA (alkylating agents)
3. Interfere with cell division, destroying completed DNA and inhibiting transcription of RNA (antitumor antibiotics)
4. Act nonselectively on cancer cells (nitrosoureas)
 IV. Investigational Agents and Protocols
IV. Investigational Agents and ProtocolsA. Overview
1. Clinical investigation of new antineoplastic agents is accomplished in four phases
2. The agent becomes commercially available after successful completion of Phase III
B. Phases and Goals of Clinical Trials
1. Phase I: To determine the pharmacokinetics of the agent, the maximum tolerated dose, the dose range, and dose-limiting toxicities; about 20 to 80 participants
2. Phase II: To determine the antitumor activity for a specific type of cancer or cancers; to determine the common short-term side effects, risks, and safety; about 100 to 300 participants
3. Phase III: To compare the new agent(s) with the usual treatment protocol(s), to confirm the effectiveness and monitor the side effects; about 1,000 to 3,000 participants; after the Phase III trial, FDA approval is sought
4. Phase IV: To determine the risks, benefits, optimal use, and long-term safety in post-marketing studies
C. Components of a Clinical Trial Protocol
1. Background information about the study
2. Objectives
3. Patient selection criteria
4. Schema (identifies arms of the study and regimens to be tested)
5. Toxicity information
6. Required laboratory and diagnostic procedures
7. Evaluation parameters
8. Informed consent
9. References
10. Resource person and contact information
D. Institutional Review Board (IRB)
1. All investigational agents, studies, and protocols must be approved by an IRB
2. The objective of the IRB is to protect human participants and to oversee the informed consent process
3. Membership and actions of the IRB are determined and monitored by federal regulations
E. Informed Consent
1. Risks, benefits, purpose, length of the study, and required procedures are explained prior to participant signing the agreement
2. Patient may withdraw from the study at any time
F. Policies and Procedures
1. Organizational policies and procedures must be followed
2. National standards are references for the development of the procedures
G. Nursing Responsibilities in Clinical Trials
1. Ensure informed consent
2. Follow protocol precisely
3. Monitor patient per protocol
4. Documentation
a. Record toxicities according to severity scale
b. Record response to therapy
5. Support patient
 V. Preadministration Considerations
V. Preadministration ConsiderationsA. Patient History
1. Medical history
a. Cardiac, respiratory, renal, hepatic, and GI
b. Other conditions (e.g., diabetes mellitus)
c. Allergies: food, drug, bee stings, and latex
1) Patients with allergies to bees are at increased risk for sensitivity reactions to chemotherapy agents and biotherapies
d. Prior history of cancer and cancer treatment: chemotherapy, radiation therapy, biotherapy, hormonal therapy, complementary, and/or alternative treatment
2. Surgical history
a. Procedures related to cancer diagnosis (e.g., hemicolectomy for colon cancer)
b. Other surgical procedures
3. Menstrual history and pregnancies
4. Psychosocial history
a. Psychiatric history
b. Marital status and children
c. Current living situation (home, apartment, nursing home, or homeless)
d. Transportation needs
e. Financial issues
f. Need for referrals (social services and community resources)
g. Usual coping strategies
5. Medication use and compliance
a. Current prescribed medications: scheduled and as needed
b. Over-the-counter medications
c. Herbs
d. Vitamins
e. Other (alternative drugs)
f. Street drugs
6. Culture, language, and religion
a. Preferred language for receiving medical information
b. Need for translator
c. Cultural and/or religious practices that may influence care
7. Understanding of disease and anticipated treatment
B. Clinical Assessment
1. Overview
a. The patient is assessed before each treatment
b. Focused assessments are related to systems potentially affected by each agent in the treatment protocol
2. Cardiovascular
a. Blood pressure abnormalities
b. Apical and radial pulse: heart sounds and irregularities
c. Chest pain, diaphoresis, and palpitations
3. GI
a. Constipation
b. Diarrhea
c. Jaundice
d. Mucositis
e. Nausea
f. Taste alterations
g. Vomiting
h. Weight loss
4. Genitourinary
a. Urine color and amount
b. Dysuria, frequency, and pain
5. Integumentary
a. Alopecia
b. Cellulitis
c. Desquamation
d. Erythema
e. Facial flushing
f. Hyperpigmentation
g. Nail ridges
h. Pain
i. Phlebitis
j. Pruritus
k. Radiosensitization
l. Rash
6. Neurologic
a. Alterations in sensorium
b. Ataxia
c. Hearing loss
d. Paresthesia: numbness, tingling in fingers and toes
e. Tinnitus
f. Concentration and memory deficits
g. Fatigue
7. Respiratory
a. Breath sounds
b. Respirations
c. Edema
d. Pulse oximetry
e. Cough: dry or productive
f. Dyspnea
8. Hepatic
a. Pain
b. Jaundice
c. Ascites
9. Pain
a. Location
b. Severity (pain scale)
c. Precipitating factors
d. Relieving factors
C. Evaluation of Diagnostic Findings Prior to Each Treatment
1. Complete blood count (CBC) with differential; most chemotherapeutic agents cause myelosuppression
a. White blood count (WBC)
1) Absolute neutrophil count (ANC) is the basis for knowing a patient’s risk for infection
2) Normal neutrophil count ranges from 3,000 to 7,000 cells/mm3
3) To calculate ANC: WBC × (% polys [segs] + % bands) Example: WBC = 1,200; segs = 0.35%; bands = 0.05% 1,200 × 0.4 = 480
4) Neutropenia = ANC < 1,500 cells/mm3; ANC < 500 considered severe; high risk for infection
5) Treatment usually held if neutropenia exists
b. Platelet count
1) Normal is 150,000 to 400,000 cells/mm3
2) Low platelet count increases the risk for bleeding
3) Treatment may be held or dose decreased if thrombocytopenia present
c. Red blood cell (RBC) count
1) Normal RBC: 4.0 to 6.2 × 1012/L for adults
2) Normal hemoglobin: 13.6 to 18 g/dL for males; 12 to 16 g/dL for females
3) Normal hematocrit: 37% to 51% for males; 35% to 47% for females
4) A blood transfusion and/or erythropoietin may be given for severe anemia
2. Blood urea nitrogen (BUN) and creatinine
a. To evaluate the renal function
b. An elevated creatinine may require a change in therapy or a dose reduction
c. The goal is to prevent renal failure from renal toxic agents
3. Liver function studies
a. May help to determine diagnosis
b. Used to monitor treatment
4. Tumor markers
a. Baseline obtained
b. Evaluated during treatment to monitor the response to treatment
c. Monitored at the completion of the treatment and at follow-up visits to determine reoccurrence or progression of disease
5. Pulmonary function study
a. Baseline obtained for agents potentially causing pulmonary toxicities
b. Ordered periodically to monitor treatment with these agents
6. Cardiac evaluation
a. Baseline obtained prior to administration of agents with potential to cause cardiac abnormalities
b. Obtained periodically to monitor the effects of treatment
D. Understanding the Disease and Goals of Treatment
1. Type of cancer
a. Solid tumor (e.g., lung cancer)
b. Hematologic cancer (e.g., lymphoma)
c. Stage of disease
1) Reported in pathology report
2) Determined by staging system
3) Most common is TNM staging system
a) T = size of the tumor
b) N = lymph node involvement
c) M = metastasis
2. Goals of the treatment
a. Cure: disease-free; a complete response that lasts 5 years or more
b. Control: increased survival or prevention of metastatic disease
c. Palliation: reduced severity or alleviation of symptoms with or without reduction in tumor burden and a goal to provide comfort
E. Evaluation of Treatment Orders
1. Treatment is determined by the oncologist based on tumor type, stage, patient’s performance status, and number of times patient previously treated
2. Protocol prescribed
a. Single-agent chemotherapy: one agent used
b. Combination chemotherapy
1) Most commonly used
2) More than one agent is used to increase the response
3) Provides multiple mechanisms of action with synergistic activity
4) Helps to prevent drug resistance
c. Dose calculations
1) Pediatric doses are usually calculated by weight (kg)
2) Most agents’ doses are calculated by body surface area (BSA)
a) Require patient’s measured height and weight
b) Several formulas and methods of calculation; follow institution policy
c) Most common formula is the Mosteller Equation:
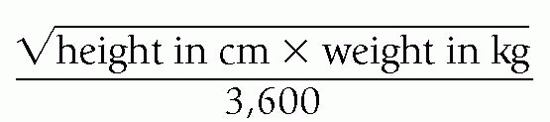
d) Reputable online BSA calculators or those found in an electronic medical record may be used instead of the formula
e) Dose is calculated as BSA × dose expressed in m2 (example: drug prescribed is doxorubicin 50 mg/m2; patient’s BSA is 1.8; 50 × 1.8 = 90 mg of doxorubicin)
3) Area under the curve (AUC)
a) Used to calculate Carboplatin dose
b) Does not use BSA
c) Creatinine clearance must first be calculated
d) Dosing calculations include the patient’s age, sex, weight, glomerular filtration rate, and creatinine clearance
e) Computer-based programs can be used for calculations
d. Check orders for appropriate premedications given before agent(s) based on anticipated side effects of the therapy such as antiemetics, antihistamines, steroids, electrolytes, vitamins, and fluids
e. Ensure licensed independent practitioner’s (LIP’s) orders include, but not limited to the following, post-treatment medications such as antiemetics, steroids, electrolytes, and antipyretics
f. Identify the antineoplastic drugs that are irritants and vesicants before administration
1) Overview
a) Agents are classified by the degree of irritation or tissue damage that is caused if infiltrated
b) Includes the categories of vesicants and irritants; agents not listed in either category are presumed to be nondamaging if infiltrated
c) Careful vein and device selection with frequent assessment during administration is necessary
2) Vesicants
a) Toxic to soft tissue, inducing tissue necrosis if extravasation occurs
b) Interventions
Central vascular access device (CVAD) is recommended for administration of vesicants
Avoid using peripheral VAD that is more than 24 hours old
Monitor the infusion site during administration of vesicant drugs; check patency every 2 to 5 mL with IV push vesicant administration
If there is an actual or suspected extravasation, stop administration of drug and fluids and initiate extravasation protocol
Instruct patient regarding appropriate follow-up for evaluation of tissue damage and possible referral to a plastic surgeon
3) Irritants
a) Burning, pain, edema, or erythema may occur without soft tissue necrosis
F. Vascular Access Considerations
1. Peripheral access appropriate for short-term treatment, nonvesicant agents, in patients with sufficient useable veins
2. Central vascular access appropriate for long-term treatments, vesicant agents, in patients with few useable veins and in those with contraindications for access such as arm affected by cerebral vascular accident (CVA), side of a mastectomy with lymph node dissection, or arm with dialysis fistula
G. Patient and Caregiver Education
1. Determine the preferred language for education; elicit the assistance of a translator as needed
2. Determine the preferred learning style
3. Determine from the patient the information desired
4. Provide verbal and written information
5. Involve a relative, friend, or caregiver if possible
6. Review the plan of care
a. Agents to be used
b. Route(s) of administration
c Number of cycles
d. Frequency of treatment
e. Tests/procedures required before and during the treatment to monitor the response
7. Provide information on the following:
a. Name of agents—generic and brand names
b. Potential side effects
c. Managing symptoms
1) Prescribed medications and use
2) Self-care strategies
a) Infection and bleeding precautions
b) Mouth care
c) Avoid exposure to sun
d) Avoid aspirin and aspirin-containing products
e) Adequate fluid intake; nutritional support
f) Energy conservation strategies
g) Home management of antiemetic and antidiarrheal medications
h) When to call the LIP
i) Body fluid precautions
j) Precautions related to sexual intercourse and contraception
d. Available support services such as dietician, social services, physical therapy, psychology services, and community resources
e. Follow-up appointment and laboratory test monitoring
1) Routine laboratory tests before treatments
2) Laboratory tests, radiological studies, and other tests required to determine the response to treatment, measure degree of toxicity, or to restage disease
8. Patient rights: treatment refusal
9. Follow-up care
10. When and how to contact the healthcare team
 VI. Administration Considerations
VI. Administration ConsiderationsA. Personnel
1. An RN administering chemotherapy must have the required knowledge, skill, and competency per organizational policy
2. Policies and procedures are followed for handling, administration, and disposal by appropriate personnel
B. Vascular Access
1. Peripheral
a. Nonvesicant medications for short duration
b. May be used for intravenous (IV) push administration of vesicant medications
2. Short-term CVADs
a. Short-term therapy, usually 30 to 60 days
b. Nontunneled short-term CVAD (e.g., subclavian or jugular catheter)
c. Peripherally inserted central catheter
3. Long-term CVADs
a. Used when extended therapy is expected
b. Patients with limited venous access, anticipated long-term treatment, or those receiving vesicants or irritating chemotherapy
c. Tunneled CVAD
d. Implanted vascular access port
1) Note that there is risk of potential needle dislodgement with port-based infusions, which increases the risk of infiltration/extravasation
C. Routes of Administration
1. Systemic
a. IV, most common
b. Subcutaneous
c. Intramuscular
d. Oral
2. Regional
a. Intra-arterial
b. Intraventricular or intrathecal
c. Intraperitoneal
d. Intrapleural
e. Intravesicular
f. Topical
D. Methods of IV Administration
1. Direct IV push—agent given directly into the catheter without a diluent or IV fluids
2. IV push through free-flowing IV—agent given through the injection port on the administration set closest to the patient while IV fluids are flowing freely to dilute the agent during administration
3. Continuous infusion—agent given over several hours to days
4. Intermittent infusion—a short-term infusion (e.g., 30 to 60 minutes)
E. Compounding
1. Limit to qualified personnel who have demonstrated competency to compound drugs under a biologic safety cabinet
2. Specific equipment
a. Class II biologic vertical laminar airflow safety cabinet
b. Syringes with luer-lock connections
c. Vented vials
F. Specific Considerations for Administration
1. LIP orders
a. Verify medication, dose, route, and patient identification
b. Double-check orders and medication with another nurse or a pharmacist
2. Recommendations when administering chemotherapy agents
a. Use safe and protective techniques to minimize the potential risk
b. Follow the Occupational Safety and Health Administration (OSHA) guidelines for handling chemotherapy
1) Wear disposable, powder-free nitrile gloves
2) Change gloves at regular intervals and when integrity is compromised
3) Wear closed-front gowns with long sleeves and fitted cuffs; fabric of low permeability material
3. Maintain aseptic technique
4. Assure patency of vascular access device
a. Administer 0.9% sodium chloride before antineoplastic given and observe for signs of infiltration
b. Instruct patient to report any burning or pain during infusion
c. Assess patency by periodic check of blood return
G. Immediate Complications
1. Hypersensitivity reactions
a. An allergic reaction mediated by the immune system
b. Reaction usually occurs within minutes of initiating the infusion
c. Follow hypersensitivity protocol
2. Infusion reactions
a. A reaction due to cytokine release from targeted cells and other immune cells
b. Associated with the administration of monoclonal antibodies (see Part II)
3. Extravasation
a. Infiltration of a vesicant
b. Tissue damage and necrosis usually develops
c. Symptoms include pain at the catheter insertion site, redness, swelling, burning, and absence of blood return
d. Follow extravasation protocol
4. Vein irritation
a. Inflammation of a peripheral vein by an irritant agent
b. Produces redness or discoloration of vein, aching, and tight feeling along the vein
c. Blood return present
5. Vein flare
a. Course of vein becomes reddened
b. Blood return is present
c. Subsides when the agent is stopped
d. Hydrocortisone is given IV to diminish the reaction
 VII. Side Effects/Toxicities
VII. Side Effects/ToxicitiesA. Neutropenia (hematologic)
1. Decreased WBCs with decreased neutrophils, predisposing patient to risk of infection; ANC < 1,500
2. Interventions
a. Assess patient for signs of infection: fever (>38°C or >100.4°F), chills, sore throat, cough, dyspnea, frequency or urgency with voiding, edema, or purulent drainage at sites of skin breaks
b. Monitor WBC and ANC
c. After chemotherapy, the point at which the lowest blood cell count is reached is called the nadir
1) Usually about 7 to 10 days after chemotherapy administration
2) WBC and platelet counts are usually the first to drop
d. Notify the LIP of WBC < 4,000/mm3 or ANC < 2,000/mm3
e. Obtain cultures and chest X-ray, administer antibiotics as ordered
f. Administer colony-stimulating factors (G-CSF or GM-CSF) to prevent neutropenia
g. Teach patient and visitors regarding hand hygiene
h. Remove plants and flowers from patient’s immediate environment
i. Use automatic ice dispensers versus ice bins
j. Instruct patient to avoid contact with animal contaminants such as droppings, feces, urine, saliva, and litter box
B. Thrombocytopenia (hematologic)
1. Decreased platelet count; platelets <100,000 mm3
2. Interventions
a. Assess patient for bleeding in gums and nose, presence of blood in urine and feces, easy bruising of skin, headaches, hypotension, tachycardia, or enlarged spleen/liver
b. Monitor platelet count and notify LIP if <50,000/mm3
c. Instruct patient regarding bleeding precautions, including the use of electric razors and soft toothbrush and avoiding nose blowing
d. Caution against activities that may cause abrasions, cuts, or trauma
e. Apply pressure to the sites of bleeding and notify the LIP if bleeding persists
C. Anemia (hematologic)
1. Reduced number of RBCs with a decrease in the blood’s oxygen-carrying capacity
a. Normal hemoglobin: 13.6 to 18 g/dL for males; 12 to 16 g/dL for females
b. Normal hematocrit: 37% to 51% for males; 35% to 47% for females
2. Interventions
a. Assess patient for fatigue, dyspnea, pallor, tinnitus, tachycardia, palpitations, fainting, headaches, and irritability
b. Monitor hemoglobin and hematocrit and notify the LIP of abnormal results
c. Administer RBCs or oxygen as ordered; or erythropoietin (Epogen) as ordered
d. Instruct the patient regarding the need for rest and planning of activities to maximize energy
D. Nausea and Vomiting (gastrointestinal)
1. Increased risk of dehydration and compromised nutritional status
2. Interventions
a. Monitor oral intake
b. Provide adequate antiemetic therapy based on emetogenic properties of the antineoplastic therapy
c. Provide information on nutrition and nutritional supplements
d. Administer antiemetics
1) Dexamethasone (Decadron)
a) Administer a 10- to 20-mg single dose 30 minutes prior to chemotherapy
b) Give in combination with other medications
2) Lorazepam (Ativan)
a) Administer 1 to 3 mg by mouth or 0.5 to 2.5 mg IV (IV push or through injection port of IV administration set over 1 minute)
b) Give in combination with other medications
3) Metoclopramide (Reglan)
a) Administer 2 to 3 mg/kg IV by infusion over at least 15 minutes, 30 minutes prior to chemotherapy
b) Give in combination with other medications
4) Ondansetron (Zofran)
a) Administer a single dose of 8 to 32 mg IV over 15 minutes, 30 minutes prior to chemotherapy
5) Granisetron (Kytril)
a) Administer a 0.01-mg/kg IV dose over 5 minutes, 30 minutes prior to chemotherapy
6) Palonosetron (Aloxi)
a) Administer a single dose of 0.25 mg IV push over 30 seconds, 30 minutes prior to chemotherapy
7) Aprepitant and Fosaprepitant (Emend)
a) Aprepitant is given orally 30 minutes prior to chemotherapy
b) Fosaprepitant is a single 150 mg IV dose given 30 minutes prior to chemotherapy
8) Prochlorperazine (Compazine)
a) Given by mouth, IV, per rectum, as needed for nausea
E. Anorexia (gastrointestinal)
1. Increased risk of compromised nutritional status
2. Interventions
a. Monitor patient’s weight
b. Monitor laboratory values (serum albumin, nitrogen balance, glucose, transferrin, and electrolytes)
c. Instruct patient to eat foods high in protein and calories, use nutritional supplements, and try eating smaller, more frequent meals
d. Stress the importance of oral care and identify the methods to stimulate taste buds and appetite
e. Consider appetite stimulants (e.g., Megace) or parenteral nutrition if gastrointestinal function is altered
F. Constipation (gastrointestinal)
1. Decreased bowel elimination
2. Interventions
a. Assess bowel elimination patterns, diet and fluid intake, and medication use
b. Assess patient for dry skin and mucous membranes, for poor skin turgor, and for bowel sounds
c. Instruct patient regarding increasing fluid intake, eating high-fiber and high-bulk foods, and the appropriate use of laxatives or stool softeners, as indicated
G. Diarrhea (gastrointestinal)
1. Increased risk of fluid and electrolyte disturbance and dehydration
2. Interventions
a. Assess the frequency and character of stools
b. Monitor patient’s weight
c. Monitor serum electrolytes
d. Administer antidiarrheal agents as ordered
e. Instruct patient regarding low residue, high-protein diet and to eat small meals with high fluid intake at frequent intervals
f. Advise avoiding caffeine and tobacco
H. Alopecia (integumentary)
1. Increased risk of depression from poor self-image
2. Interventions
a. Instruct patient regarding the use of wigs or turbans prior to hair loss
b. Provide psychological reassurance and encourage the expression of feelings
c. For mild-to-moderate hair loss, instruct patient regarding the use of mild shampoo, gentle brushing, and avoiding hair dryers, curling irons, and curlers
I. Mucositis (integumentary)
1. Increased risk of compromised nutritional status, sepsis, and pain
2. Interventions
a. Assess oral mucosa for bleeding, erythema, ulceration, and white or yellow plaques
b. Instruct patient about good oral care with saline mouth rinse and after-meal teeth cleaning with soft toothbrush or soft swab
c. Avoid commercial mouthwashes, elective dental work, alcohol, and tobacco
d. Encourage soft, bland, and medium temperature foods and fluids
e. Instruct patient regarding the use of topical anesthetics and swish-and-swallow medications
f. For severe mucositis, provide adequate analgesics for pain
J. Cardiotoxicity
1. Risk of myocardial damage, decreased cardiac output, and tissue perfusion leading to congestive heart failure (CHF), cardiac arrhythmias, or cardiomyopathy
2. Interventions
a. Assess patient for ankle edema, cough, cyanosis, dyspnea, decreased peripheral pulses, jugular vein distention, rales, and tachycardia
b. Monitor ECG, cardiac enzymes and electrolytes, and vital signs with apical pulse
c. Notify the LIP of irregularities and administer medication as ordered
K. Neurotoxicity
1. May be central or peripheral
2. Interventions
a. Assess patient for paresthesias, deep tendon reflexes, jaw pain, bowel sounds, numbness in extremities, inability to walk on heels, confusion, or slurred speech
b. Instruct patient to take adequate fluids and use stool softeners
c. Contact physical therapy, if needed, to assist with mobility, exercise, and physical aids
L. Ototoxicity
1. Risk of decreased or lost hearing; hearing loss is more commonly seen in pediatric patients
2. Interventions
a. Assess patient for changes in hearing, sense of coordination, and tinnitus
b. Implement strategies to provide communication in the event of any hearing loss
M. Nephrotoxicity
1. Risk of impaired renal function
2. Interventions
a. Adequate hydration using IV fluids before and after drug administration; encourage oral fluid intake
b. Assess for any difficulty, frequency, or urgency with voiding
c. Monitor BUN, serum creatinine, creatinine clearance, urinalysis, and uric acid
d. Notify the LIP of any abnormal results
e. Encourage patient to empty bladder every 4 hours, especially at night
f. Monitor urine pH if patient is receiving high-dose methotrexate
N. Pulmonary Toxicity
1. Risk of decreased pulmonary function
2. Interventions
a. Assess breath sounds; observe for dry, hacking cough, and signs of dyspnea
b. Notify the LIP of abnormal findings and administer oxygen, as ordered
O. Altered Sexuality/Reproductive Function
1. Impaired sexuality, decreased quality of life, and infertility
2. Interventions
a. Assess sexual functioning and refer for consultation, as needed
b. Discuss possible reproductive dysfunction and fertility options before the start of chemotherapy (e.g. sperm banking)
c. Discuss birth control methods to use while receiving antineoplastic therapy
P. Ocular Toxicity
1. Visual impairment, infection, inflammation, and irritation
2. Interventions
a. Monitor for ocular symptoms (e.g. erythema, edema, exudate, ptosis, photophobia, pain, dryness, excessive tearing, and changes in acuity)
b. Refer patient to ophthalmologist yearly and when signs of toxicity are noted
c. Eye drops or lubricants may be prescribed to decrease inflammation, dryness, or infection
 VIII. Pharmacologic Categories
VIII. Pharmacologic CategoriesA. Alkylating Agents
1. Drug specificity: cell cycle-nonspecific
2. Action: interferes with DNA replication
3. Toxicity: hematologic, GI, renal, and reproductive
B. Antitumor Antibiotics
1. Drug specificity: cell cycle-nonspecific
2. Action: interferes with RNA synthesis, inhibits DNA synthesis by binding or reacting to DNA
3. Toxicity: hematologic, GI, reproductive, and cardiac (cumulative doses)
C. Antimetabolites
1. Drug specificity: cell cycle-specific: S phase
2. Action: inhibits DNA synthesis and protein synthesis
3. Toxicity: hematologic and GI
D. Nitrosoureas
1. Drug specificity: cell cycle-nonspecific
2. Action: interferes with DNA replication and repair
3. Toxicity: hematologic and GI
E. Vinca Alkaloids
1. Drug specificity: cell cycle-specific: G2 and M phases
2. Action: blocks DNA production and prevents cell division
3. Toxicity: neurologic
F. Miscellaneous
1. Drug specificity, action, and toxicity vary
 IX. Antineoplastic Agents
IX. Antineoplastic AgentsDosing: unless otherwise specified, the drug may be used as a single agent or in combination with other agents; the dose is individualized, based upon tolerance of and response to agent and in accordance with published protocols or literature
A. Arsenic Trioxide (Trisonox)
1. Classification
a. Pharmacologic category: miscellaneous
b. Drug specificity: cell cycle-nonspecific
c. Category: nonvesicant
2. Indications: acute promyelocytic leukemia (APL), myelodysplastic syndrome, and multiple myeloma
3. Method of administration: intermittent infusion
4. Side effects/toxicities: myelosuppression, nausea, vomiting, diarrhea, constipation, GI hemorrhage, arthralgias, bone pain, dizziness, headache, insomnia, paresthesias, pruritus, hyperpigmentation, urticaria, dyspnea, APL differentiation syndrome (fever, weight gain, dyspnea, pulmonary, or pleural infiltrates), cardiac toxicity, hypersensitivity, renal toxicity, and hepatic toxicity
5. Considerations
a. A human carcinogen
b. ECG should be done prior to treatment
B. Asparaginase (Elspar)
1. Classification
a. Pharmacologic category: miscellaneous
b. Drug specificity: cell cycle-specific
c. Category: nonvesicant
2. Indications: acute lymphocytic leukemia
3. Method of administration: intramuscular, IV push, and continuous infusion
4. Side effects/toxicities: nausea, vomiting, hypersensitivity reactions including anaphylaxis, myelosuppression, hepatic dysfunction, hyperglycemia, hyperlipidemia, onset of pancreatitis in children, lethargy, and somnolence
5. Considerations
a. Perform intradermal test dose before initial drug administration
b. Have emergency supportive care available in the event of an anaphylactic response
C. Azacytidine (Vidaza)
1. Classification
a. Pharmacologic category: antimetabolite
b. Drug specificity: cell cycle-nonspecific
c. Category: nonvesicant
2. Indications: myelodysplasia
3. Method of administration: intermittent infusion; subcutaneous
4. Side effects/toxicities: myelosuppression, nausea, vomiting, anorexia, stomatitis, constipation, diarrhea, fever, fatigue, arthralgias, and injection site irritation
5. Considerations
a. Dose modifications based on myelosuppression, renal function, and electrolytes
b. For subcutaneous administration, divide doses greater than 4 mL into two syringes
c. Must be given within 1 hour of reconstitution
D. Bendamustine (Treanda)
1. Classification
a. Pharmacologic category: alkylating agent
b. Drug specificity: cell cycle-nonspecific
c. Category: nonvesicant
2. Indications: chronic lymphocytic leukemia (CLL), indolent B-cell non-Hodgkin’s lymphoma
3. Method of administration: intermittent infusion
4. Side effects/toxicities: myelosuppression, infusion reaction, anaphylaxis, tumor lysis syndrome, nausea, vomiting, diarrhea, and rash
5. Considerations
a. Dose reductions for myelosuppression
b. Severe skin reactions, severe infusion reactions, or anaphylaxis require discontinuation of the drug
E. Bleomycin (Blenoxane)
1. Classification
a. Pharmacologic category: antitumor antibiotic
b. Drug specificity: cell cycle-nonspecific
c. Category: irritant
2. Indications: Hodgkin’s disease; non-Hodgkin’s lymphoma (NHL); squamous cell carcinoma of the head and neck; cancer of penis, cervix, vulva, and testes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
